NPC1 plays a role in the trafficking of specific cargo to melanosomes
- PMID: 37423302
- PMCID: PMC10407747
- DOI: 10.1016/j.jbc.2023.105024
NPC1 plays a role in the trafficking of specific cargo to melanosomes
Abstract
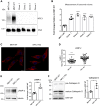
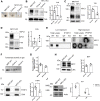
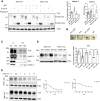
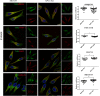
Niemann-Pick type C1 (NPC1) protein is a multimembrane spanning protein of the lysosome limiting membrane that facilitates intracellular cholesterol and sphingolipid transport. Loss-of-function mutations in the NPC1 protein cause Niemann-Pick disease type C1, a lysosomal storage disorder characterized by the accumulation of cholesterol and sphingolipids within lysosomes. To investigate whether the NPC1 protein could also play a role in the maturation of the endolysosomal pathway, here, we have investigated its role in a lysosome-related organelle, the melanosome. Using a NPC1-KO melanoma cell model, we found that the cellular phenotype of Niemann-Pick disease type C1 is associated with a decreased pigmentation accompanied by low expression of the melanogenic enzyme tyrosinase. We propose that the defective processing and localization of tyrosinase, occurring in the absence of NPC1, is a major determinant of the pigmentation impairment in NPC1-KO cells. Along with tyrosinase, two other pigmentation genes, tyrosinase-related protein 1 and Dopachrome-tautomerase have lower protein levels in NPC1 deficient cells. In contrast with the decrease in pigmentation-related protein expression, we also found a significant intracellular accumulation of mature PMEL17, the structural protein of melanosomes. As opposed to the normal dendritic localization of melanosomes, the disruption of melanosome matrix generation in NPC1 deficient cells causes an accumulation of immature melanosomes adjacent to the plasma membrane. Together with the melanosomal localization of NPC1 in WT cells, these findings suggest that NPC1 is directly involved in tyrosinase transport from the trans-Golgi network to melanosomes and melanosome maturation, indicating a novel function for NPC1.
Keywords: NPC1; Niemann-Pick disease type C; PMEL17; endosomes; lysosome-related organelles; melanosomes biogenesis; traffic; tyrosinase.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Wheeler S., Sillence D.J. Niemann–Pick type C disease: cellular pathology and pharmacotherapy. J. Neurochem. 2020;153:674–692. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

