Nanobodies with cross-neutralizing activity provide prominent therapeutic efficacy in mild and severe COVID-19 rodent models
- PMID: 37423308
- PMCID: PMC10590698
- DOI: 10.1016/j.virs.2023.07.003
Nanobodies with cross-neutralizing activity provide prominent therapeutic efficacy in mild and severe COVID-19 rodent models
Abstract
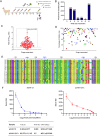
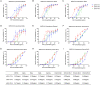
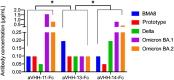
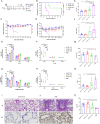
The weakened protective efficacy of COVID-19 vaccines and antibodies caused by SARS-CoV-2 variants presents a global health emergency, which underscores the urgent need for universal therapeutic antibody intervention for clinical patients. Here, we screened three alpacas-derived nanobodies (Nbs) with neutralizing activity from twenty RBD-specific Nbs. The three Nbs were fused with the Fc domain of human IgG, namely aVHH-11-Fc, aVHH-13-Fc and aVHH-14-Fc, which could specifically bind RBD protein and competitively inhibit the binding of ACE2 receptor to RBD. They effectively neutralized SARS-CoV-2 pseudoviruses D614G, Alpha, Beta, Gamma, Delta, and Omicron sub-lineages BA.1, BA.2, BA.4, and BA.5 and authentic SARS-CoV-2 prototype, Delta, and Omicron BA.1, BA.2 strains. In mice-adapted COVID-19 severe model, intranasal administration of aVHH-11-Fc, aVHH-13-Fc and aVHH-14-Fc effectively protected mice from lethal challenges and reduced viral loads in both the upper and lower respiratory tracts. In the COVID-19 mild model, aVHH-13-Fc, which represents the optimal neutralizing activity among the above three Nbs, effectively protected hamsters from the challenge of SARS-CoV-2 prototype, Delta, Omicron BA.1 and BA.2 by significantly reducing viral replication and pathological alterations in the lungs. In structural modeling of aVHH-13 and RBD, aVHH-13 binds to the receptor-binding motif region of RBD and interacts with some highly conserved epitopes. Taken together, our study illustrated that alpaca-derived Nbs offered a therapeutic countermeasure against SARS-CoV-2, including those Delta and Omicron variants which have evolved into global pandemic strains.
Keywords: Broad-spectrum; COVID-19; Nanobody; Rodent models; SARS-CoV-2; Therapeutic.
Copyright © 2023 The Authors. Publishing services by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that there are no competing interests.
Figures



Similar articles
-
Hetero-bivalent nanobodies provide broad-spectrum protection against SARS-CoV-2 variants of concern including Omicron.Cell Res. 2022 Sep;32(9):831-842. doi: 10.1038/s41422-022-00700-3. Epub 2022 Jul 29. Cell Res. 2022. PMID: 35906408 Free PMC article.
-
A Novel Nanobody Targeting Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Receptor-Binding Domain Has Potent Cross-Neutralizing Activity and Protective Efficacy against MERS-CoV.J Virol. 2018 Aug 29;92(18):e00837-18. doi: 10.1128/JVI.00837-18. Print 2018 Sep 15. J Virol. 2018. PMID: 29950421 Free PMC article.
-
A Glycosylated RBD Protein Induces Enhanced Neutralizing Antibodies against Omicron and Other Variants with Improved Protection against SARS-CoV-2 Infection.J Virol. 2022 Sep 14;96(17):e0011822. doi: 10.1128/jvi.00118-22. Epub 2022 Aug 16. J Virol. 2022. PMID: 35972290 Free PMC article.
-
Passive Immunotherapy Against SARS-CoV-2: From Plasma-Based Therapy to Single Potent Antibodies in the Race to Stay Ahead of the Variants.BioDrugs. 2022 May;36(3):231-323. doi: 10.1007/s40259-022-00529-7. Epub 2022 Apr 27. BioDrugs. 2022. PMID: 35476216 Free PMC article. Review.
-
SARS-CoV-2 neutralizing antibody bebtelovimab - a systematic scoping review and meta-analysis.Front Immunol. 2023 Aug 28;14:1100263. doi: 10.3389/fimmu.2023.1100263. eCollection 2023. Front Immunol. 2023. PMID: 37701439 Free PMC article.
Cited by
-
SARS-CoV-2 Specific Nanobodies Neutralize Different Variants of Concern and Reduce Virus Load in the Brain of h-ACE2 Transgenic Mice.Viruses. 2024 Jan 25;16(2):185. doi: 10.3390/v16020185. Viruses. 2024. PMID: 38399961 Free PMC article.
-
Research Progress on the Application of Neutralizing Nanobodies in the Prevention and Treatment of Viral Infections.Microorganisms. 2025 Jun 11;13(6):1352. doi: 10.3390/microorganisms13061352. Microorganisms. 2025. PMID: 40572239 Free PMC article. Review.
-
Respiratory delivery of passive immunotherapies for SARS-CoV-2 prophylaxis and therapy.Hum Vaccin Immunother. 2023 Aug;19(2):2260040. doi: 10.1080/21645515.2023.2260040. Epub 2023 Oct 6. Hum Vaccin Immunother. 2023. PMID: 37799070 Free PMC article. Review.
-
The Use of Heterologous Antigens for Biopanning Enables the Selection of Broadly Neutralizing Nanobodies Against SARS-CoV-2.Antibodies (Basel). 2025 Mar 7;14(1):23. doi: 10.3390/antib14010023. Antibodies (Basel). 2025. PMID: 40136472 Free PMC article.
-
A surrogate BSL2-compliant infection model recapitulating key aspects of human Marburg virus disease.Emerg Microbes Infect. 2025 Dec;14(1):2449083. doi: 10.1080/22221751.2024.2449083. Epub 2025 Jan 12. Emerg Microbes Infect. 2025. PMID: 39745141 Free PMC article.
References
-
- Altarawneh H.N., Chemaitelly H., Ayoub H.H., Tang P., Hasan M.R., Yassine H.M., Al-Khatib H.A., Smatti M.K., Coyle P., Al-Kanaani Z., Al-Kuwari E., Jeremijenko A., Kaleeckal A.H., Latif A.N., Shaik R.M., Abdul-Rahim H.F., Nasrallah G.K., Al-Kuwari M.G., Butt A.A., Al-Romaihi H.E., Al-Thani M.H., Al-Khal A., Bertollini R., Abu-Raddad L.J. Effects of previous infection and vaccination on symptomatic omicron infections. N. Engl. J. Med. 2022;387:21–34. - PMC - PubMed
-
- Baig A.M. Targeting neuroinvasion by SARS-CoV-2: emerging trends in drug and antibody delivery to combat covid-19. ACS Chem. Neurosci. 2021;12:2555–2557. - PubMed
-
- Barnes C.O., Jette C.A., Abernathy M.E., Dam K.A., Esswein S.R., Gristick H.B., Malyutin A.G., Sharaf N.G., Huey-Tubman K.E., Lee Y.E., Robbiani D.F., Nussenzweig M.C., West A.P., Jr., Bjorkman P.J. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588:682–687. - PMC - PubMed
-
- Baum A., Fulton B.O., Wloga E., Copin R., Pascal K.E., Russo V., Giordano S., Lanza K., Negron N., Ni M., Wei Y., Atwal G.S., Murphy A.J., Stahl N., Yancopoulos G.D., Kyratsous C.A. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020;369:1014–1018. - PMC - PubMed
-
- Boudewijns R., Thibaut H.J., Kaptein S.J.F., Li R., Vergote V., Seldeslachts L., Van Weyenbergh J., De Keyzer C., Bervoets L., Sharma S., Liesenborghs L., Ma J., Jansen S., Van Looveren D., Vercruysse T., Wang X., Jochmans D., Martens E., Roose K., De Vlieger D., Schepens B., Van Buyten T., Jacobs S., Liu Y., Martí-Carreras J., Vanmechelen B., Wawina-Bokalanga T., Delang L., Rocha-Pereira J., Coelmont L., Chiu W., Leyssen P., Heylen E., Schols D., Wang L., Close L., Matthijnssens J., Van Ranst M., Compernolle V., Schramm G., Van Laere K., Saelens X., Callewaert N., Opdenakker G., Maes P., Weynand B., Cawthorne C., Vande Velde G., Wang Z., Neyts J., Dallmeier K. Stat2 signaling restricts viral dissemination but drives severe pneumonia in SARS-CoV-2 infected hamsters. Nat. Commun. 2020;11:5838. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

