High-Pressure Processing of Human Milk: A Balance between Microbial Inactivation and Bioactive Protein Preservation
- PMID: 37423385
- PMCID: PMC10517232
- DOI: 10.1016/j.tjnut.2023.07.001
High-Pressure Processing of Human Milk: A Balance between Microbial Inactivation and Bioactive Protein Preservation
Abstract
Background: Donor human milk banks use Holder pasteurization (HoP; 62.5°C, 30 min) to reduce pathogens in donor human milk, but this process damages some bioactive milk proteins.
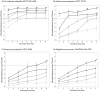
Objectives: We aimed to determine minimal parameters for high-pressure processing (HPP) to achieve >5-log reductions of relevant bacteria in human milk and how these parameters affect an array of bioactive proteins.
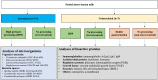
Methods: Pooled raw human milk inoculated with relevant pathogens (Enterococcus faecium, Staphylococcus aureus, Listeria monocytogenes, Cronobacter sakazakii) or microbial quality indicators (Bacillus subtilis and Paenibacillus spp. spores) at 7 log CFU/mL was processed at 300-500 MPa at 16-19°C (due to adiabatic heating) for 1-9 min. Surviving microbes were enumerated using standard plate counting methods. For raw milk, and HPP-treated and HoP-treated milk, the immunoreactivity of an array of bioactive proteins was assessed via ELISA and the activity of bile salt-stimulated lipase (BSSL) was determined via a colorimetric substrate assay.
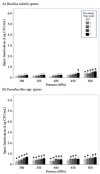
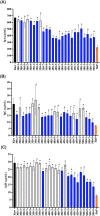
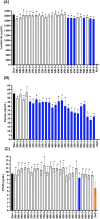
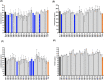
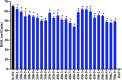
Results: Treatment at 500 MPa for 9 min resulted in >5-log reductions of all vegetative bacteria, but <1-log reduction in B. subtilis and Paenibacillus spores. HoP decreased immunoglobulin A (IgA), immunoglobulin M (IgM), immunoglobulin G, lactoferrin, elastase and polymeric immunoglobulin receptor (PIGR) concentrations, and BSSL activity. The treatment at 500 MPa for 9 min preserved more IgA, IgM, elastase, lactoferrin, PIGR, and BSSL than HoP. HoP and HPP treatments up to 500 MPa for 9 min caused no losses in osteopontin, lysozyme, α-lactalbumin and vascular endothelial growth factor.
Conclusion: Compared with HoP, HPP at 500 MPa for 9 min provides >5-log reduction of tested vegetative neonatal pathogens with improved retention of IgA, IgM, lactoferrin, elastase, PIGR, and BSSL in human milk.
Keywords: antibody; breast milk; enzyme; infant nutrition; microbial inactivation; mother’s milk; premature infant; preterm infant; protease; thermal processing.
Copyright © 2023 American Society for Nutrition. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Comparison of the Effect of Holder Pasteurization and High-Pressure Processing on Human Milk Bacterial Load and Bioactive Factors Preservation.J Pediatr Gastroenterol Nutr. 2021 May 1;72(5):756-762. doi: 10.1097/MPG.0000000000003065. J Pediatr Gastroenterol Nutr. 2021. PMID: 33847290 Free PMC article.
-
Digestion of human milk processed by high pressure processing and Holder pasteurization using a dynamic in vitro model of the preterm infant.Food Chem. 2023 Jun 15;411:135477. doi: 10.1016/j.foodchem.2023.135477. Epub 2023 Jan 11. Food Chem. 2023. PMID: 36701922
-
Bile Salt-Stimulated Lipase Activity in Donor Breast Milk Influenced by Pasteurization Techniques.Front Nutr. 2020 Nov 12;7:552362. doi: 10.3389/fnut.2020.552362. eCollection 2020. Front Nutr. 2020. PMID: 33282897 Free PMC article.
-
Human Milk Composition and Preservation: Evaluation of High-pressure Processing as a Nonthermal Pasteurization Technology.Crit Rev Food Sci Nutr. 2016;56(6):1043-60. doi: 10.1080/10408398.2012.753402. Crit Rev Food Sci Nutr. 2016. PMID: 25313944 Review.
-
Breast milk preservation: thermal and non-thermal processes and their effect on microorganism inactivation and the content of bioactive and nutritional compounds.Front Nutr. 2024 Feb 22;10:1325863. doi: 10.3389/fnut.2023.1325863. eCollection 2023. Front Nutr. 2024. PMID: 38455872 Free PMC article. Review.
Cited by
-
Impact of high-pressure processing on the bioactive compounds of milk - A comprehensive review.J Food Sci Technol. 2024 Sep;61(9):1632-1651. doi: 10.1007/s13197-024-05938-w. Epub 2024 Mar 6. J Food Sci Technol. 2024. PMID: 39049911 Free PMC article. Review.
-
Optimized Ultraviolet-C Processing Inactivates Pathogenic and Spoilage-Associated Bacteria while Preserving Bioactive Proteins, Vitamins, and Lipids in Human Milk.J Agric Food Chem. 2024 May 29;72(21):12198-12208. doi: 10.1021/acs.jafc.4c02120. Epub 2024 May 16. J Agric Food Chem. 2024. PMID: 38752986 Free PMC article.
References
-
- Arslanoglu S., Ziegler E.E., Moro G.E. World Association of Perinatal Medicine Working Group on Nutrition. Donor human milk in preterm infant feeding: evidence and recommendations. J. Perinat. Med. 2010;38(4):347–351. - PubMed
-
- Menon G., Williams T.C. Human milk for preterm infants: why, what, when and how? Arch. Dis. Child Fetal. Neonatal. Ed. 2013;98(6):F559–F562. - PubMed
-
- Vohr B.R., Poindexter B.B., Dusick A.M., McKinley L.T., Higgins R.D., Langer J.C. Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics. 2007;120(4):e953–e959. - PubMed
-
- Singhal A., Cole T.J., Lucas A. Early nutrition in preterm infants and later blood pressure: two cohorts after randomised trials. Lancet. 2001;357(9254):413–419. - PubMed
-
- Beck K.L., Weber D., Phinney B.S., Smilowitz J.T., Hinde K., Lönnerdal B. Comparative proteomics of human and macaque milk reveals species-specific nutrition during postnatal development. J. Proteome. Res. 2015;14(5):2143–2157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

