Stop and go signals at the stigma-pollen interface of the Brassicaceae
- PMID: 37423711
- PMCID: PMC10517188
- DOI: 10.1093/plphys/kiad301
Stop and go signals at the stigma-pollen interface of the Brassicaceae
Erratum in
-
Correction to: Stop and go signals at the stigma-pollen interface of the Brassicaceae.Plant Physiol. 2024 Jul 31;195(4):3138. doi: 10.1093/plphys/kiae242. Plant Physiol. 2024. PMID: 38717872 Free PMC article. No abstract available.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures

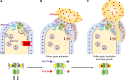
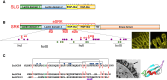
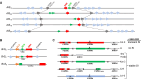
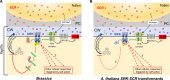
References
-
- Bateman AJ. Self-incompatibility systems in angiosperms. III. Cruciferae. Heredity. 1955:9(1):53–68. 10.1038/hdy.1955.2 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

