Testing pharmacotherapies for alcohol use disorder with cue exposure paradigms: A systematic review and quantitative synthesis of human laboratory trial methodology
- PMID: 37423771
- PMCID: PMC12127972
- DOI: 10.1111/acer.15143
Testing pharmacotherapies for alcohol use disorder with cue exposure paradigms: A systematic review and quantitative synthesis of human laboratory trial methodology
Abstract
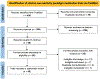
Alcohol cue exposure is a widely used experimental paradigm for screening pharmacotherapies for alcohol use disorder (AUD). Medication-related reductions in cue-reactivity signal early efficacy and inform medications development. Yet, across trials, the design of cue exposure, parameter testing, and outcome reporting is heterogeneous. This systematic review is a quantitative synthesis of trial methodologies and effect size estimation for AUD medication-related craving and psychophysiological outcomes under the cue exposure paradigm. A PubMed search was conducted on January 3, 2022 based on identified pharmacotherapies for peer-reviewed articles reported in English. Study-level characteristics, including sample descriptors, paradigm design, analytic approach, and Cochrane Risk of Bias, along with descriptive statistics for cue-exposure outcomes, were coded by two independent raters. Study-level effect sizes were estimated for craving and psychophysiological outcomes separately and sample-level effect sizes were calculated for each medication. Thirty-six trials, comprising 1640 participants and testing 19 different medications satisfied eligibility criteria. All studies reported on biological sex (71% male participants on average). The exposure paradigms implemented used in vivo (n = 26), visual (n = 8), and audio script (n = 2) cues. Some trials included means for craving by medication condition in text (k = 7) or figures (k = 18). The quantitative synthesis included 63 effect sizes (craving kes = 47; psychophysiological kes = 16) from 28 unique randomized trials testing 15 medications for effects on cue reactivity. For cue-induced craving, eight medications (kes range: 1-12) demonstrated small-to-medium effects (Cohen's d range: |0.24-0.64|) compared to placebo, with individuals randomized to receive medication reporting lower craving following cue exposure. Recommendations are provided to promote further consilience, so that the utility of cue exposure paradigms can be maximized in the development of effective AUD pharmacotherapies. Future work should explore the predictive utility of medication-related reductions in cue-reactivity on clinical outcomes.
Keywords: alcohol use disorder; cue-reactivity; medications; pharmacotherapy; systematic review.
© 2023 by the Research Society on Alcohol.
Conflict of interest statement
Figures
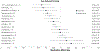
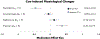


Similar articles
-
Leveraging meta-regression to test if medication effects on cue-induced craving are associated with clinical efficacy.Psychopharmacology (Berl). 2024 Aug;241(8):1679-1689. doi: 10.1007/s00213-024-06589-7. Epub 2024 Apr 13. Psychopharmacology (Berl). 2024. PMID: 38613685 Free PMC article.
-
Are medication effects on subjective response to alcohol and cue-induced craving associated? A meta regression study.Psychopharmacology (Berl). 2023 Sep;240(9):1921-1930. doi: 10.1007/s00213-023-06409-4. Epub 2023 Jul 15. Psychopharmacology (Berl). 2023. PMID: 37452887 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Do behavioral pharmacology findings predict clinical trial outcomes? A proof-of-concept in medication development for alcohol use disorder.Neuropsychopharmacology. 2021 Feb;46(3):519-527. doi: 10.1038/s41386-020-00913-3. Epub 2020 Nov 24. Neuropsychopharmacology. 2021. PMID: 33235284 Free PMC article.
-
Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: An Updated Systematic Review for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2018 Sep. Report No.: 18-05239-EF-1. PMID: 30354042 Free Books & Documents. Review.
Cited by
-
A Secondary Analysis Suggests That Repetitive Transcranial Magnetic Stimulation Applied to the Left Dorsolateral Prefrontal Cortex Reduces Cue-Induced-Craving in Treatment Seeking Participants with Cannabis Use Disorder.medRxiv [Preprint]. 2025 Jan 17:2025.01.16.25320690. doi: 10.1101/2025.01.16.25320690. medRxiv. 2025. PMID: 39867364 Free PMC article. Preprint.
-
Leveraging meta-regression to test if medication effects on cue-induced craving are associated with clinical efficacy.Psychopharmacology (Berl). 2024 Aug;241(8):1679-1689. doi: 10.1007/s00213-024-06589-7. Epub 2024 Apr 13. Psychopharmacology (Berl). 2024. PMID: 38613685 Free PMC article.
-
A practice quit model to test early efficacy of medications for alcohol use disorder in a randomized clinical trial.Psychopharmacology (Berl). 2024 Mar;241(3):543-553. doi: 10.1007/s00213-023-06504-6. Epub 2023 Nov 28. Psychopharmacology (Berl). 2024. PMID: 38012333 Free PMC article. Clinical Trial.
-
Characterizing alcohol cue reactive and non-reactive individuals with alcohol use disorder.Addict Behav. 2024 Aug;155:108028. doi: 10.1016/j.addbeh.2024.108028. Epub 2024 Apr 10. Addict Behav. 2024. PMID: 38640885 Free PMC article.
-
Translating medication effects for alcohol use disorder across preclinical, human laboratory, and clinical trial outcomes using meta-analysis.Transl Psychiatry. 2025 Jul 21;15(1):250. doi: 10.1038/s41398-025-03473-6. Transl Psychiatry. 2025. PMID: 40691154 Free PMC article.
References
-
- Anton RF, Moak DH & Latham PK 1996. The obsessive compulsive drinking scale: A new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry, 53(3), pp 225–31. - PubMed
-
- Bach P, Reinhard I, Koopmann A, Bumb JM, Sommer WH, Vollstadt-Klein S & Kiefer F 2022. Test-retest reliability of neural alcohol cue-reactivity: Is there light at the end of the magnetic resonance imaging tube? Addict Biol, 27(1), pp e13069. - PubMed
-
- Bormann I. 2012. DigitizeIt. Version 2.0 ed. http://www.digitizeit.de/.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

