Enantiopure Dinaphtho[2,3-b:2,3-f]thieno[3,2-b]thiophenes: Reaching High Magnetoresistance Effect in OFETs
- PMID: 37424043
- PMCID: PMC10502826
- DOI: 10.1002/advs.202301914
Enantiopure Dinaphtho[2,3-b:2,3-f]thieno[3,2-b]thiophenes: Reaching High Magnetoresistance Effect in OFETs
Abstract
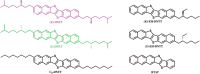
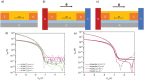
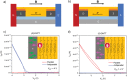
Chiral molecules are known to behave as spin filters due to the chiral induced spin selectivity (CISS) effect. Chirality can be implemented in molecular semiconductors in order to study the role of the CISS effect in charge transport and to find new materials for spintronic applications. In this study, the design and synthesis of a new class of enantiopure chiral organic semiconductors based on the well-known dinaphtho[2,3-b:2,3-f]thieno[3,2-b]thiophene (DNTT) core functionalized with chiral alkyl side chains is presented. When introduced in an organic field-effect transistor (OFET) with magnetic contacts, the two enantiomers, (R)-DNTT and (S)-DNTT, show an opposite behavior with respect to the relative direction of the magnetization of the contacts, oriented by an external magnetic field. Each enantiomer displays an unexpectedly high magnetoresistance over one preferred orientation of the spin current injected from the magnetic contacts. The result is the first reported OFET in which the current can be switched on and off upon inversion of the direction of the applied external magnetic field. This work contributes to the general understanding of the CISS effect and opens new avenues for the introduction of organic materials in spintronic devices.
Keywords: chiral induced spin selectivity effect; chirality; magnetoresistance; organic semiconductors; spin; transistors.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Enantiopure 2-(2-ethylhexyl)dinaphtho[2,3-b:2',3'-f]thieno[3,2-b]thiophenes: synthesis, single-crystal structure and a surprising lack of influence of stereoisomerism on thin-film structure and electronic properties.Mater Horiz. 2022 Jan 4;9(1):444-451. doi: 10.1039/d1mh01119g. Mater Horiz. 2022. PMID: 34788783
-
Diphenyl derivatives of dinaphtho[2,3-b:2',3'-f]thieno[3,2-b]thiophene: organic semiconductors for thermally stable thin-film transistors.ACS Appl Mater Interfaces. 2013 Apr 10;5(7):2331-6. doi: 10.1021/am3026163. Epub 2013 Feb 14. ACS Appl Mater Interfaces. 2013. PMID: 23410846
-
Organic semiconductors based on [1]benzothieno[3,2-b][1]benzothiophene substructure.Acc Chem Res. 2014 May 20;47(5):1493-502. doi: 10.1021/ar400282g. Epub 2014 May 1. Acc Chem Res. 2014. PMID: 24785263
-
The Importance of Spin State in Chiral Supramolecular Electronics.Front Chem. 2021 Aug 4;9:722727. doi: 10.3389/fchem.2021.722727. eCollection 2021. Front Chem. 2021. PMID: 34422770 Free PMC article. Review.
-
Chirality-Induced Spin Selectivity in Hybrid Organic-Inorganic Perovskite Semiconductors.Annu Rev Phys Chem. 2025 Apr;76(1):519-537. doi: 10.1146/annurev-physchem-082423-032933. Epub 2025 Feb 14. Annu Rev Phys Chem. 2025. PMID: 39952641 Review.
Cited by
-
On the importance of crystal structures for organic thin film transistors.Acta Crystallogr C Struct Chem. 2024 Oct 1;80(Pt 10):601-611. doi: 10.1107/S2053229624008283. Epub 2024 Sep 4. Acta Crystallogr C Struct Chem. 2024. PMID: 39226426 Free PMC article.
-
Mechanism for Electrostatically Generated Magnetoresistance in Chiral Systems without Spin-Dependent Transport.ACS Nano. 2024 Feb 27;18(8):6028-6037. doi: 10.1021/acsnano.3c12925. Epub 2024 Feb 14. ACS Nano. 2024. PMID: 38353652 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
