Tethering ATG16L1 or LC3 induces targeted autophagic degradation of protein aggregates and mitochondria
- PMID: 37424101
- PMCID: PMC10549199
- DOI: 10.1080/15548627.2023.2234797
Tethering ATG16L1 or LC3 induces targeted autophagic degradation of protein aggregates and mitochondria
Abstract
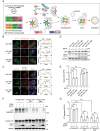
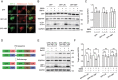
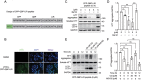
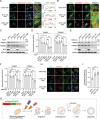
Proteolysis-targeting chimeras (PROTACs) based on the ubiquitin-proteasome system have made great progress in the field of drug discovery. There is mounting evidence that the accumulation of aggregation-prone proteins or malfunctioning organelles is associated with the occurrence of various age-related neurodegenerative disorders and cancers. However, PROTACs are inefficient for the degradation of such large targets due to the narrow entrance channel of the proteasome. Macroautophagy (hereafter referred to as autophagy) is known as a self-degradative process involved in the degradation of bulk cytoplasmic components or specific cargoes that are sequestered into autophagosomes. In the present study, we report the development of a generalizable strategy for the targeted degradation of large targets. Our results suggested that tethering large target models to phagophore-associated ATG16L1 or LC3 induced targeted autophagic degradation of the large target models. Furthermore, we successfully applied this autophagy-targeting degradation strategy to the targeted degradation of HTT65Q aggregates and mitochondria. Specifically, chimeras consisting of polyQ-binding peptide 1 (QBP) and ATG16L1-binding peptide (ABP) or LC3-interacting region (LIR) induced targeted autophagic degradation of pathogenic HTT65Q aggregates; and the chimeras consisting of mitochondria-targeting sequence (MTS) and ABP or LIR promoted targeted autophagic degradation of dysfunctional mitochondria, hence ameliorating mitochondrial dysfunction in a Parkinson disease cell model and protecting cells from apoptosis induced by the mitochondrial stress agent FCCP. Therefore, this study provides a new strategy for the selective proteolysis of large targets and enrich the toolkit for autophagy-targeting degradation.Abbreviations: ABP: ATG16L1-binding peptide; ATG16L1: autophagy related 16 like 1; ATTEC: autophagy-tethering compound; AUTAC: autophagy-targeting chimera; AUTOTAC: autophagy-targeting chimera; Baf A1: bafilomycin A1; BCL2: BCL2 apoptosis regulator; CALCOCO2/NDP52: calcium binding and coiled-coil domain 2; CASP3: caspase 3; CPP: cell-penetrating peptide; CQ: chloroquine phosphate; DAPI: 4',6-diamidino-2-phenylindole; DCM: dichloromethane; DMF: N,N-dimethylformamide; DMSO: dimethyl sulfoxide; EBSS: Earle's balanced salt solution; FCCP: carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone; FITC: fluorescein-5-isothiocyanate; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GFP: green fluorescent protein; HEK293: human embryonic kidney 293; HEK293T: human embryonic kidney 293T; HPLC: high-performance liquid chromatography; HRP: horseradish peroxidase; HTT: huntingtin; LIR: LC3-interacting region; MAP1LC3/LC3: microtubule associated protein 1 light chain 3; MFF: mitochondrial fission factor; MTS: mitochondria-targeting sequence; NBR1: NBR1 autophagy cargo receptor; NLRX1: NLR family member X1; OPTN: optineurin; P2A: self-cleaving 2A peptide; PB1: Phox and Bem1p; PBS: phosphate-buffered saline; PE: phosphatidylethanolamine; PINK1: PTEN induced kinase 1; PRKN: parkin RBR E3 ubiquitin protein ligase; PROTACs: proteolysis-targeting chimeras; QBP: polyQ-binding peptide 1; SBP: streptavidin-binding peptide; SDS-PAGE: sodium dodecyl sulfate-polyacrylamide gel electrophoresis; SPATA33: spermatogenesis associated 33; TIMM23: translocase of inner mitochondrial membrane 23; TMEM59: transmembrane protein 59; TOMM20: translocase of outer mitochondrial membrane 20; UBA: ubiquitin-associated; WT: wild type.
Keywords: ATG16L1; HTT65Q aggregates; LC3; autophagy-targeting degradation; mitochondria; proteolysis-targeting chimeras.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
