Whole-brain monosynaptic inputs to lateral periaqueductal gray glutamatergic neurons in mice
- PMID: 37424163
- PMCID: PMC10651995
- DOI: 10.1111/cns.14338
Whole-brain monosynaptic inputs to lateral periaqueductal gray glutamatergic neurons in mice
Abstract
Objective: The lateral periaqueductal gray (LPAG), which mainly contains glutamatergic neurons, plays an important role in social responses, pain, and offensive and defensive behaviors. Currently, the whole-brain monosynaptic inputs to LPAG glutamatergic neurons are unknown. This study aims to explore the structural framework of the underlying neural mechanisms of LPAG glutamatergic neurons.
Methods: This study used retrograde tracing systems based on the rabies virus, Cre-LoxP technology, and immunofluorescence analysis.
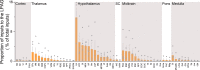
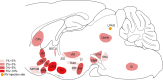
Results: We found that 59 nuclei projected monosynaptic inputs to the LPAG glutamatergic neurons. In addition, seven hypothalamic nuclei, namely the lateral hypothalamic area (LH), lateral preoptic area (LPO), substantia innominata (SI), medial preoptic area, ventral pallidum, posterior hypothalamic area, and lateral globus pallidus, projected most densely to the LPAG glutamatergic neurons. Notably, we discovered through further immunofluorescence analysis that the inputs to the LPAG glutamatergic neurons were colocalized with several markers related to important neurological functions associated with physiological behaviors.
Conclusion: The LPAG glutamatergic neurons received dense projections from the hypothalamus, especially nuclei such as LH, LPO, and SI. The input neurons were colocalized with several markers of physiological behaviors, which show the pivotal role of glutamatergic neurons in the physiological behaviors regulation by LPAG.
Keywords: glutamatergic neurons; lateral periaqueductal gray; monosynaptic inputs; rabies virus.
© 2023 The Authors. CNS Neuroscience & Therapeutics Published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare no conflict of interest.
Figures




References
-
- Tovote P, Esposito MS, Botta P, et al. Midbrain circuits for defensive behaviour. Nature. 2016;534(7606):206‐212. - PubMed
-
- Yu H, Miao W, Ji E, et al. Social touch‐like tactile stimulation activates a tachykinin 1‐oxytocin pathway to promote social interactions. Neuron. 2022;110(6):1051‐1067. - PubMed
-
- Hu Z, Mu Y, Huang L, et al. A visual circuit related to the periaqueductal gray area for the antinociceptive effects of bright light treatment. Neuron. 2022;110:1712‐1727. - PubMed
-
- Gao ZR, Chen WZ, Liu MZ, et al. Tac1‐expressing neurons in the periaqueductal gray facilitate the itch‐scratching cycle via descending regulation. Neuron. 2019;101(1):45‐59. - PubMed
-
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17(9):379‐389. - PubMed
Publication types
MeSH terms
Grants and funding
- 2022YFA1604504/The National Key Research and Development Program of China
- 2021ZD0203400/The STI 2030-major project
- 82171479/The National Natural Science Foundation of China
- 82020108014/The National Natural Science Foundation of China
- 32070984/The National Natural Science Foundation of China
- 201409001800/Shanghai Science and Technology Innovation Action Plan Laboratory Animal Research Project
- Program for Shanghai Outstanding Academic Leaders
- 2018SHZDZX01/Shanghai Municipal Science and Technology Major Project, and ZJLab, and Shanghai Center for Brain Science and Brain-inspired Technology
- LG-TKN-202203-01/Lingang Laboratory & National Key Laboratory of Human Factors Engineering Joint Grant
LinkOut - more resources
Full Text Sources

