NAD metabolism: Role in senescence regulation and aging
- PMID: 37424179
- PMCID: PMC10776128
- DOI: 10.1111/acel.13920
NAD metabolism: Role in senescence regulation and aging
Abstract
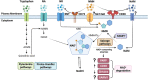
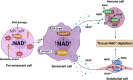
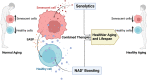
The geroscience hypothesis proposes that addressing the biology of aging could directly prevent the onset or mitigate the severity of multiple chronic diseases. Understanding the interplay between key aspects of the biological hallmarks of aging is essential in delivering the promises of the geroscience hypothesis. Notably, the nucleotide nicotinamide adenine dinucleotide (NAD) interfaces with several biological hallmarks of aging, including cellular senescence, and changes in NAD metabolism have been shown to be involved in the aging process. The relationship between NAD metabolism and cellular senescence appears to be complex. On the one hand, the accumulation of DNA damage and mitochondrial dysfunction induced by low NAD+ can promote the development of senescence. On the other hand, the low NAD+ state that occurs during aging may inhibit SASP development as this secretory phenotype and the development of cellular senescence are both highly metabolically demanding. However, to date, the impact of NAD+ metabolism on the progression of the cellular senescence phenotype has not been fully characterized. Therefore, to explore the implications of NAD metabolism and NAD replacement therapies, it is essential to consider their interactions with other hallmarks of aging, including cellular senescence. We propose that a comprehensive understanding of the interplay between NAD boosting strategies and senolytic agents is necessary to advance the field.
Keywords: NAD+ metabolism; SASP; aging; nicotinamide adenine dinucleotide; senescence.
© 2023 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
ENC holds a patent on CD38 inhibitors licensed by Elysium Health. ENC consults for Calico, Mitobridge, and Cytokinetics. Others declare no conflicts.
Figures

References
-
- Abdellatif, M. , Sedej, S. , & Kroemer, G. (2021). NAD(+) metabolism in cardiac health, aging, and disease. Circulation, 144, 1795–1817. - PubMed
-
- Abdellatif, M. , Trummer‐Herbst, V. , Koser, F. , Durand, S. , Adão, R. , Vasques‐Nóvoa, F. , Freundt, J. K. , Voglhuber, J. , Pricolo, M. R. , Kasa, M. , Türk, C. , Aprahamian, F. , Herrero‐Galán, E. , Hofer, S. J. , Pendl, T. , Rech, L. , Kargl, J. , Anto‐Michel, N. , Ljubojevic‐Holzer, S. , … Sedej, S. (2021). Nicotinamide for the treatment of heart failure with preserved ejection fraction. Sci Transl Med, 13, eabd7064. - PMC - PubMed
-
- Aflatounian, A. , Paris, V. R. , Richani, D. , Edwards, M. C. , Cochran, B. J. , Ledger, W. L. , Gilchrist, R. B. , Bertoldo, M. J. , Wu, L. E. , & Walters, K. A. (2022). Declining muscle NAD(+) in a hyperandrogenism PCOS mouse model: Possible role in metabolic dysregulation. Mol Metab, 65, 101583. - PMC - PubMed
-
- Agorrody, G. , Peclat, T. R. , Peluso, G. , Gonano, L. A. , Santos, L. , van Schooten, W. , Chini, C. C. S. , Escande, C. , Chini, E. N. , & Contreras, P. (2022). Benefits in cardiac function by CD38 suppression: Improvement in NAD+ levels, exercise capacity, heart rate variability and protection against catecholamine‐induced ventricular arrhythmias. J Mol Cell Cardiol, 166, 11–22. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

