The Effect and Safety Assessment of Metformin and DLBS3233 (A Bioactive Fraction of Lagerstroemia speciosa and Cinnamomum burmannii) on Improving Metabolic Parameters in Women with Polycystic Ovary Syndrome
- PMID: 37424700
- PMCID: PMC10329438
- DOI: 10.2147/IJWH.S409685
The Effect and Safety Assessment of Metformin and DLBS3233 (A Bioactive Fraction of Lagerstroemia speciosa and Cinnamomum burmannii) on Improving Metabolic Parameters in Women with Polycystic Ovary Syndrome
Abstract
Background: Polycystic ovary syndrome (PCOS) can lead to compensatory hyperinsulinemia with consequent metabolic abnormalities in women. In this study, DLBS3233 and Metformin were used to be tested. DLBS3233 itself is the new insulin-sensitizing drug, a combination-bioactive-fraction derived from two Indonesian herbals, Lagerstroemia speciosa and Cinnamomum burmannii. DLBS3233 alone and in combination with metformin were evaluated for efficacy and safety in insulin-resistant women with polycystic ovary syndrome (PCOS).
Methods: A randomized, double-blind, 3-arm, double-dummy, non-inferiority, and also a controlled clinical study was conducted at the Dr. Kariadi Hospital, Indonesia, between October 2014 and February 2019. The study involved 60 female subjects (with 20 female subjects in each group) that had polycystic ovary syndrome (PCOS).Treatment I consists of one placebo capsule twice per day and one 100 mg DLBS3233 capsule once per day. Treatment II consists of one placebo caplet once per day and one 750 mg Metformin XR caplet twice per day. Treatment III consists of one 750 mg Metformin XR caplet twice per day and one 100 mg DLBS3233 capsule once per day.
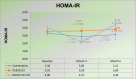
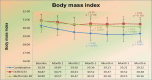
Results: In treatment I, the homeostatic model assessment for insulin resistance (HOMA-IR) levels were 3.55, 3.59, and 3.80 at pretest, 3 months, and 6 months after intervention, respectively. In treatment II, the HOMA-IR level were 4.00, 2.21, and 4.40 at pretest, 3 months, and 6 months after intervention respectively. In treatment III, the HOMA-IR levels were 3.30, 2.86, and 3.12 at pretest, 3 months, and 6 months after intervention, respectively. There was no apparent difference existed in the fasting plasma glucose (FPG), high-density lipoprotein (HDL), triglycerides, ferriman-gallwey scores (FGS), and safety assessment on vital signs and laboratory examinations (liver function and renal function) in all groups.
Conclusion: Either DLBS3233 alone or the DLBS3233/Metformin combination showed no significant efficacy and did not negatively affect cardiovascular function, liver and kidney function in PCOS subjects.
Clinicaltrialsgov identifier: NCT01999686 Date: 3rd of December, 2013.
Keywords: DLBS3233; homeostatic model assessment for insulin resistance; metabolic parameters; metformin; polycystic ovary syndrome.
© 2023 Hidayat et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
Similar articles
-
The Efficacy and Safety of DLBS3233, A Combined Bioactive Fraction of Cinnamomum burmanii and Lagerstroemia speciosa Plants on The Endocrine-Metabolic Profile of Women with Polycystic Ovary Syndrome: A Randomized Clinical Trial.Int J Fertil Steril. 2024 Jul 13;18(Suppl 1):35-47. doi: 10.22074/ijfs.2023.551350.1283. Int J Fertil Steril. 2024. PMID: 39033369 Free PMC article.
-
Insulin sensitizer in prediabetes: a clinical study with DLBS3233, a combined bioactive fraction of Cinnamomum burmanii and Lagerstroemia speciosa.Drug Des Devel Ther. 2016 Mar 29;10:1279-89. doi: 10.2147/DDDT.S97568. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27099473 Free PMC article. Clinical Trial.
-
DLBS3233, a combined bioactive fraction of Cinnamomum burmanii and Lagerstroemia speciosa, in type-2 diabetes mellitus patients inadequately controlled by metformin and other oral antidiabetic agents.J Complement Integr Med. 2016 Dec 1;13(4):413-420. doi: 10.1515/jcim-2016-0031. J Complement Integr Med. 2016. PMID: 27451997
-
Effect of metformin on clinical, metabolic and endocrine outcomes in women with polycystic ovary syndrome: a meta-analysis of randomized controlled trials.Curr Med Res Opin. 2017 Sep;33(9):1545-1557. doi: 10.1080/03007995.2017.1279597. Epub 2017 Feb 3. Curr Med Res Opin. 2017. PMID: 28058854 Review.
-
The Effect of Metformin on Polycystic Ovary Syndrome in Overweight Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Int J Endocrinol. 2020 Sep 16;2020:5150684. doi: 10.1155/2020/5150684. eCollection 2020. Int J Endocrinol. 2020. PMID: 33014044 Free PMC article. Review.
References
-
- Neven ACH, Laven J, Teede HJ, Boyle JA. A summary on polycystic ovary syndrome: diagnostic criteria, prevalence, clinical manifestations, and management according to the latest international guidelines. In: Seminars in Reproductive Medicine. Thieme Medical Publishers; 2018. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical