Computer-aided drug design approaches applied to screen natural product's structural analogs targeting arginase in Leishmania spp
- PMID: 37424744
- PMCID: PMC10323282
- DOI: 10.12688/f1000research.129943.3
Computer-aided drug design approaches applied to screen natural product's structural analogs targeting arginase in Leishmania spp
Abstract
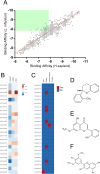
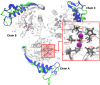
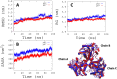
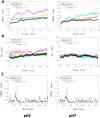
Introduction: Leishmaniasis is a disease with high mortality rates and approximately 1.5 million new cases each year. Despite the new approaches and advances to fight the disease, there are no effective therapies. Methods: Hence, this study aims to screen for natural products' structural analogs as new drug candidates against leishmaniasis. We applied Computer-aided drug design (CADD) approaches, such as virtual screening, molecular docking, molecular dynamics simulation, molecular mechanics-generalized Born surface area (MM-GBSA) binding free estimation, and free energy perturbation (FEP) aiming to select structural analogs from natural products that have shown anti-leishmanial and anti-arginase activities and that could bind selectively against the Leishmania arginase enzyme. Results: The compounds 2H-1-benzopyran, 3,4-dihydro-2-(2-methylphenyl)-(9CI), echioidinin, and malvidin showed good results against arginase targets from three parasite species and negative results for potential toxicities. The echioidinin and malvidin ligands generated interactions in the active center at pH 2.0 conditions by MM-GBSA and FEP methods. Conclusions: This work suggests the potential anti-leishmanial activity of the compounds and thus can be further in vitro and in vivo experimentally validated.
Keywords: Leishmania arginase; antiprotozoal agents; drug discovery; computer-aided drug design; leishmaniasis; molecular dynamics simulation.
Copyright: © 2023 Barazorda-Ccahuana HL et al.
Conflict of interest statement
No competing interests were disclosed.
Figures
Similar articles
-
Unveiling Novel Arginase Inhibitors for Cutaneous Leishmaniasis Using Drug Repurposing and Virtual Screening Approaches.J Cell Biochem. 2025 Aug;126(8):e70060. doi: 10.1002/jcb.70060. J Cell Biochem. 2025. PMID: 40847551
-
Exploring Type II Diabetes Inhibitors from Genus Daphne Plant-species: An Integrated Computational Study.Comb Chem High Throughput Screen. 2025;28(8):1413-1442. doi: 10.2174/0113862073262227231005074024. Comb Chem High Throughput Screen. 2025. PMID: 38584562
-
Interventions for Old World cutaneous leishmaniasis.Cochrane Database Syst Rev. 2017 Dec 1;12(12):CD005067. doi: 10.1002/14651858.CD005067.pub5. Cochrane Database Syst Rev. 2017. PMID: 29192424 Free PMC article.
-
Interventions for Old World cutaneous leishmaniasis.Cochrane Database Syst Rev. 2017 Nov 17;11(11):CD005067. doi: 10.1002/14651858.CD005067.pub4. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2017 Dec 01;12:CD005067. doi: 10.1002/14651858.CD005067.pub5. PMID: 29149474 Free PMC article. Updated.
-
Integrating traditional QSAR and read-across-based regression models for predicting potential anti-leishmanial azole compounds.Mol Divers. 2025 Aug;29(4):3207-3231. doi: 10.1007/s11030-024-11070-w. Epub 2024 Dec 10. Mol Divers. 2025. PMID: 39653961
Cited by
-
Evaluation of the Antileishmanial Activity of Some Benzimidazole Derivatives Using In Vitro and In Silico Techniques.Vet Sci. 2025 Jun 5;12(6):550. doi: 10.3390/vetsci12060550. Vet Sci. 2025. PMID: 40559787 Free PMC article.
-
Targeting with Structural Analogs of Natural Products the Purine Salvage Pathway in Leishmania (Leishmania) infantum by Computer-Aided Drug-Design Approaches.Trop Med Infect Dis. 2024 Feb 3;9(2):41. doi: 10.3390/tropicalmed9020041. Trop Med Infect Dis. 2024. PMID: 38393130 Free PMC article.
-
Exploring the Potential of Malvidin and Echiodinin as Probable Antileishmanial Agents Through In Silico Analysis and In Vitro Efficacy.Molecules. 2025 Jan 4;30(1):173. doi: 10.3390/molecules30010173. Molecules. 2025. PMID: 39795229 Free PMC article.
-
Targeting Leishmania infantum Mannosyl-oligosaccharide glucosidase with natural products: potential pH-dependent inhibition explored through computer-aided drug design.Front Pharmacol. 2024 May 30;15:1403203. doi: 10.3389/fphar.2024.1403203. eCollection 2024. Front Pharmacol. 2024. PMID: 38873424 Free PMC article.
-
Navigating the Chemical Space and Chemical Multiverse of a Unified Latin American Natural Product Database: LANaPDB.Pharmaceuticals (Basel). 2023 Sep 30;16(10):1388. doi: 10.3390/ph16101388. Pharmaceuticals (Basel). 2023. PMID: 37895859 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

