Monoclonal antibodies against lipopolysaccharide protect against Pseudomonas aeruginosa challenge in mice
- PMID: 37424774
- PMCID: PMC10326049
- DOI: 10.3389/fcimb.2023.1191806
Monoclonal antibodies against lipopolysaccharide protect against Pseudomonas aeruginosa challenge in mice
Abstract
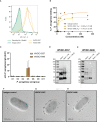
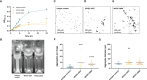
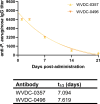
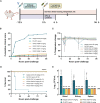
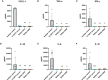
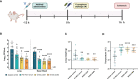
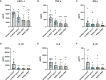
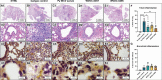
Pseudomonas aeruginosa is a common cause of hospital-acquired infections, including central line-associated bloodstream infections and ventilator-associated pneumonia. Unfortunately, effective control of these infections can be difficult, in part due to the prevalence of multi-drug resistant strains of P. aeruginosa. There remains a need for novel therapeutic interventions against P. aeruginosa, and the use of monoclonal antibodies (mAb) is a promising alternative strategy to current standard of care treatments such as antibiotics. To develop mAbs against P. aeruginosa, we utilized ammonium metavanadate, which induces cell envelope stress responses and upregulates polysaccharide expression. Mice were immunized with P. aeruginosa grown with ammonium metavanadate and we developed two IgG2b mAbs, WVDC-0357 and WVDC-0496, directed against the O-antigen lipopolysaccharide of P. aeruginosa. Functional assays revealed that WVDC-0357 and WVDC-0496 directly reduced the viability of P. aeruginosa and mediated bacterial agglutination. In a lethal sepsis model of infection, prophylactic treatment of mice with WVDC-0357 and WVDC-0496 at doses as low as 15 mg/kg conferred 100% survival against challenge. In both sepsis and acute pneumonia models of infection, treatment with WVDC-0357 and WVDC-0496 significantly reduced bacterial burden and inflammatory cytokine production post-challenge. Furthermore, histopathological examination of the lungs revealed that WVDC-0357 and WVDC-0496 reduced inflammatory cell infiltration. Overall, our results indicate that mAbs directed against lipopolysaccharide are a promising therapy for the treatment and prevention of P. aeruginosa infections.
Keywords: O5; Pseudomonas aeruginosa; ammonium metavanadate; anti-lipopolysaccharide antibody; immunotherapeutics; monoclonal antibody (mAb); pneumonia; sepsis.
Copyright © 2023 Kang, Mateu-Borrás, Monroe, Sen-Kilic, Miller, Dublin, Huckaby, Yang, Pyles, Nunley, Chapman, Amin, Damron and Barbier.
Conflict of interest statement
Authors JK, MB, and FD are inventors on a pending patent application related to the sequences of WVDC-0357 and WVDC-0496. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Akiyama M., Oishi K., Tao M., Matsumoto K., Pollack M. (2000). Antibacterial properties of Pseudomonas aeruginosa immunotype 1 lipopolysaccharide-specific monoclonal antibody (MAb) in a murine thigh infection model: combined effects of MAb and ceftazidime. Microbiol. Immunol. 44 (8), 629–635. doi: 10.1111/j.1348-0421.2000.tb02543.x - DOI - PubMed
-
- Centers for Disease Control (2019). Antibiotic Resistance Threats in the United States 2019. Atlanta, GA: U.S. Department of Health and Human Services. doi: >http://dx.doi.org/10.15620/cdc:82532 - DOI
-
- Barton H. A., Johnson Z., Cox C. D., Vasil A. I., Vasil M. L. (1996). Ferric uptake regulator mutants of Pseudomonas aeruginosa with distinct alterations in the iron-dependent repression of exotoxin a and siderophores in aerobic and microaerobic environments. Mol. Microbiol. 21 (5), 1001–1017. doi: 10.1046/j.1365-2958.1996.381426.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

