Multifunctional hybrid hydrogel for the prevention of post-surgery tumor recurrence
- PMID: 37424816
- PMCID: PMC10326567
Multifunctional hybrid hydrogel for the prevention of post-surgery tumor recurrence
Abstract
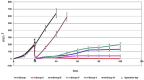
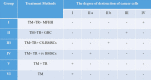
In this study, we present a multifunctional hybrid hydrogel (MFHH) for the prevention of postoperative tumor recurrence. MFHH consists of two components; component A - containing a gelatin-based cisplatin, which destroys the residual cancer after surgery, and component B - containing macroporous gelatin microcarriers (CultiSpher) loaded with freeze-dried bone marrow stem cells (BMSCs), which activates the wound healing process. We also evaluated the effects of MFHH in a subcutaneous Ehrlich tumor mouse model. MFHH acted as a local delivery system by directly supplying cisplatin to the tumor environment, resulting in excellent anti-cancer effects and minimal side effects. MFHH released cisplatin gradually to destroy the residual tumors, thereby preventing loco-regional recurrence. We have also demonstrated that BMSCs are able to inhibit residual tumor growth. Moreover, CultiSpher loaded with BMSCs acted as an injection 3D scaffold and easily filled the wound defect formed by tumor removal, and the paracrine factors of the freeze-dried BMSCs accelerated the wound healing process. The components of the MFHH can be used both separately and together. However, for the successful application of MFHH in clinical practice, it is necessary to study in more detail the role of paracrine factors of freeze-dried BMSCs in the inhibition or proliferation of residual cancer. These questions will be the focus of our future research.
Keywords: Ehrlich solid tumor; Multifunctional hybrid hydrogel; bone marrow stem cells; cisplatin mechanism of action; local drug delivery systems; macroporous gelatin microcarriers (CultiSpher).
AJCR Copyright © 2023.
Conflict of interest statement
None.
Figures



Similar articles
-
Local drug delivery system for the treatment of tongue squamous cell carcinoma in rats.Oncol Lett. 2022 Jan;23(1):13. doi: 10.3892/ol.2021.13131. Epub 2021 Nov 11. Oncol Lett. 2022. PMID: 34820012 Free PMC article.
-
3D printed heterogeneous hybrid hydrogel scaffolds for sequential tumor photothermal-chemotherapy and wound healing.Biomater Sci. 2022 Sep 27;10(19):5648-5661. doi: 10.1039/d2bm00903j. Biomater Sci. 2022. PMID: 35994007
-
Effect of kartogenin-loaded gelatin methacryloyl hydrogel scaffold with bone marrow stimulation for enthesis healing in rotator cuff repair.J Shoulder Elbow Surg. 2021 Mar;30(3):544-553. doi: 10.1016/j.jse.2020.06.013. Epub 2020 Jul 7. J Shoulder Elbow Surg. 2021. PMID: 32650072
-
[Influence of the stiffness of three-dimensionally bioprinted extracellular matrix analogue on the differentiation of bone mesenchymal stem cells into skin appendage cells].Zhonghua Shao Shang Za Zhi. 2020 Nov 20;36(11):1013-1023. doi: 10.3760/cma.j.cn501120-20200811-00375. Zhonghua Shao Shang Za Zhi. 2020. PMID: 33238684 Chinese.
-
[Research progresses of paracrine effect of bone marrow derived mesenchymal stem cells on wound healing].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2012 Oct;29(5):999-1002. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2012. PMID: 23198449 Review. Chinese.
References
-
- Endo M, Lin PP. Surgical margins in the management of extremity soft tissue sarcoma. Chin Clin Oncol. 2018;7:37. - PubMed
-
- Graeser M, Schrading S, Gluz O, Strobel K, Herzog C, Umutlu L, Frydrychowicz A, Rjosk-Dendorfer D, Würstlein R, Culemann R, Eulenburg C, Adams J, Nitzsche H, Prange A, Kümmel S, Grischke EM, Forstbauer H, Braun M, Potenberg J, von Schumann R, Aktas B, Kolberg-Liedtke C, Harbeck N, Kuhl CK, Nitz U. Magnetic resonance imaging and ultrasound for prediction of residual tumor size in early breast cancer within the ADAPT subtrials. Breast Cancer Res. 2021;23:36. - PMC - PubMed
LinkOut - more resources
Full Text Sources
