Combination of systemic immune-inflammation index and albumin-bilirubin grade predict prognosis of regorafenib in unresectable hepatocellular carcinoma
- PMID: 37424826
- PMCID: PMC10326579
Combination of systemic immune-inflammation index and albumin-bilirubin grade predict prognosis of regorafenib in unresectable hepatocellular carcinoma
Abstract
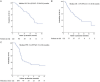
Regorafenib improved prognosis for unresectable hepatocellular carcinoma (uHCC) after sorafenib treatment failure. We aimed to investigate prognostic value of combining systemic inflammatory markers with liver function evaluation in patients receiving sorafenib-regorafenib sequential therapy. A total of 122 uHCC patients who received sorafenib-regorafenib sequential therapy were retrospectively enrolled for analysis. The pre-treatment preserving liver function and six inflammatory indexes were collected. The Cox regression model was used to identify independent predictors of progression-free survival (PFS) and overall survival (OS). Baseline ALBI grade I (hazard ratio (HR) = 0.725, P = 0.040 for PFS; HR = 0.382, P = 0.012 for OS) and systemic inflammatory index (SII) ≤ 330 (HR = 0.341, P = 0.017 for OS; HR = 0.485, P = 0.037 for OS) were identified as independent prognostic factors in multivariable analysis and were used to develop the scoring system. Patients who fulfilled both criteria (2 points; score-high) had the longest median PFS (not-reached) and OS (not-reached), followed by fulfilling 1 criterion (1 point; score-intermediate; PFS: 3.7 months and OS: 17.9 months), and patients fulfilled no criterion (0 point; score-low; PFS: 2.9 months, overall log-rank P = 0.001 and OS: 7.5 months, overall log-rank P = 0.003). Additionally, best radiological response was significantly higher in patients with score-high (complete response/partial response/stable disease/progressive disease: score-high: 5.9%/5.9%/58.8%/29.4% vs. score-intermediate: 0%/14.0%/44.2%/41.9% vs. score-low: 0%/0%/25.0%/75.0%; P = 0.011). In conclusion, a combination of baseline ALBI grade and SII index can be used as a simple and powerful parameter to predict prognosis of uHCC patients receiving regorafenib after sorafenib-refractory treatment. The score may help with patient counseling but requires prospective validation.
Keywords: ALBI grade; Hepatocellular carcinoma; prognosis; sequential therapy; systemic inflammatory index.
AJCR Copyright © 2023.
Conflict of interest statement
None.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. - PMC - PubMed
-
- Yau T, Hsu C, Kim TY, Choo SP, Kang YK, Hou MM, Numata K, Yeo W, Chopra A, Ikeda M, Kuromatsu R, Moriguchi M, Chao Y, Zhao H, Anderson J, Cruz CD, Kudo M. Nivolumab in advanced hepatocellular carcinoma: sorafenib-experienced Asian cohort analysis. J Hepatol. 2019;71:543–552. - PubMed
-
- Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. - PubMed
-
- Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. - PubMed
LinkOut - more resources
Full Text Sources
