The suppression of cervical cancer ferroptosis by macrophages: The attenuation of ALOX15 in cancer cells by macrophages-derived exosomes
- PMID: 37425043
- PMCID: PMC10326300
- DOI: 10.1016/j.apsb.2023.03.025
The suppression of cervical cancer ferroptosis by macrophages: The attenuation of ALOX15 in cancer cells by macrophages-derived exosomes
Abstract
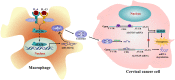
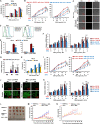
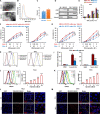
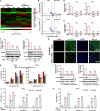
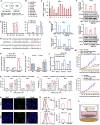
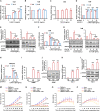
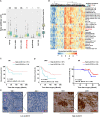
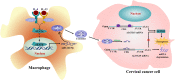
Induction of cancer cell ferroptosis has been proposed as a potential treatment in several cancer types. Tumor-associated macrophages (TAMs) play a key role in promoting tumor malignant progression and therapy resistance. However, the roles and mechanisms of TAMs in regulating tumor ferroptosis is still unexplored and remains enigmatic. This study shows ferroptosis inducers has shown therapeutic outcomes in cervical cancer in vitro and in vivo. TAMs have been found to suppress cervical cancer cells ferroptosis. Mechanistically, macrophage-derived miRNA-660-5p packaged into exosomes are transported into cancer cells. In cancer cells, miRNA-660-5p attenuates ALOX15 expression to inhibit ferroptosis. Moreover, the upregulation of miRNA-660-5p in macrophages depends on autocrine IL4/IL13-activated STAT6 pathway. Importantly, in clinical cervical cancer cases, ALOX15 is negatively associated with macrophages infiltration, which also raises the possibility that macrophages reduce ALOX15 levels in cervical cancer. Moreover, both univariate and multivariate Cox analyses show ALOX15 expression is independent prognostic factor and positively associated with good prognosis in cervical cancer. Altogether, this study reveals the potential utility of targeting TAMs in ferroptosis-based treatment and ALOX15 as prognosis indicators for cervical cancer.
Keywords: ALOX15; Cervical cancer; Exosome; Ferroptosis; Macrophages infiltration; STAT6; Tumor-associated macrophage; miRNA-660-5p.
© 2023 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures
References
-
- Cohen P.A., Jhingran A., Oaknin A., Denny L. Cervical cancer. Lancet. 2019;393:169–182. - PubMed
-
- Rose P.G., Bundy B.N., Watkins E.B., Thigpen J.T., Deppe G., Maiman M.A., et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. - PubMed
-
- Ferrandina G., Lauriola L., Distefano M.G., Zannoni G.F., Gessi M., Legge F., et al. Increased cyclooxygenase-2 expression is associated with chemotherapy resistance and poor survival in cervical cancer patients. J Clin Oncol. 2002;20:973–981. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

