Functional biomaterials for comprehensive periodontitis therapy
- PMID: 37425066
- PMCID: PMC10326309
- DOI: 10.1016/j.apsb.2022.10.026
Functional biomaterials for comprehensive periodontitis therapy
Abstract
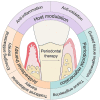
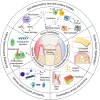
Periodontitis is an inflammatory disease caused by bacterial infection directly, and the dysregulation of host immune-inflammatory response finally destroys periodontal tissues. Current treatment strategies for periodontitis mainly involve mechanical scaling/root planing (SRP), surgical procedures, and systemic or localized delivery of antimicrobial agents. However, SRP or surgical treatment alone has unsatisfactory long-term effects and is easy to relapse. In addition, the existing drugs for local periodontal therapy do not stay in the periodontal pocket long enough and have difficulties in maintaining a steady, effective concentration to obtain a therapeutic effect, and continuous administration always causes drug resistance. Many recent studies have shown that adding bio-functional materials and drug delivery systems upregulates the therapeutic effectiveness of periodontitis. This review focuses on the role of biomaterials in periodontitis treatment and presents an overview of antibacterial therapy, host modulatory therapy, periodontal regeneration, and multifunctional regulation of periodontitis therapy. Biomaterials provide advanced approaches for periodontal therapy, and it is foreseeable that further understanding and applications of biomaterials will promote the development of periodontal therapy.
Keywords: Anti-inflammation; Anti-oxidant; Antibacterial effect; Biomaterial; Drug delivery; Host modulation; Periodontal regeneration; Periodontitis therapy.
© 2023 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Figures




References
-
- Peres M.A., Macpherson L.M.D., Weyant R.J., Daly B., Venturelli R., Mathur M.R., et al. Oral diseases: a global public health challenge. Lancet. 2019;394:249–260. - PubMed
-
- Tonetti M.S., Van Dyke T.E., on behalf of Working Group 1 of the Joint EFP/AAP Workshop Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013;40:S24–S29. - PubMed
-
- Tonetti M.S., D'Aiuto F., Nibali L., Donald A., Storry C., Parkar M., et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–920. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

