Photodynamic therapy, priming and optical imaging: Potential co-conspirators in treatment design and optimization - a Thomas Dougherty Award for Excellence in PDT paper
- PMID: 37425217
- PMCID: PMC10327884
- DOI: 10.1142/s1088424620300098
Photodynamic therapy, priming and optical imaging: Potential co-conspirators in treatment design and optimization - a Thomas Dougherty Award for Excellence in PDT paper
Abstract
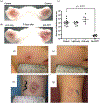
Photodynamic therapy is a photochemistry-based approach, approved for the treatment of several malignant and non-malignant pathologies. It relies on the use of a non-toxic, light activatable chemical, photosensitizer, which preferentially accumulates in tissues/cells and, upon irradiation with the appropriate wavelength of light, confers cytotoxicity by generation of reactive molecular species. The preferential accumulation however is not universal and, depending on the anatomical site, the ratio of tumor to normal tissue may be reversed in favor of normal tissue. Under such circumstances, control of the volume of light illumination provides a second handle of selectivity. Singlet oxygen is the putative favorite reactive molecular species although other entities such as nitric oxide have been credibly implicated. Typically, most photosensitizers in current clinical use have a finite quantum yield of fluorescence which is exploited for surgery guidance and can also be incorporated for monitoring and treatment design. In addition, the photodynamic process alters the cellular, stromal, and/or vascular microenvironment transiently in a process termed photodynamic priming, making it more receptive to subsequent additional therapies including chemo- and immunotherapy. Thus, photodynamic priming may be considered as an enabling technology for the more commonly used frontline treatments. Recently, there has been an increase in the exploitation of the theranostic potential of photodynamic therapy in different preclinical and clinical settings with the use of new photosensitizer formulations and combinatorial therapeutic options. The emergence of nanomedicine has further added to the repertoire of photodynamic therapy's potential and the convergence and co-evolution of these two exciting tools is expected to push the barriers of smart therapies, where such optical approaches might have a special niche. This review provides a perspective on current status of photodynamic therapy in anti-cancer and anti-microbial therapies and it suggests how evolving technologies combined with photochemically-initiated molecular processes may be exploited to become co-conspirators in optimization of treatment outcomes. We also project, at least for the short term, the direction that this modality may be taking in the near future.
Keywords: antimicrobial PDT; combination therapies; immunogenic cell death; optical imaging; photodynamic priming; photodynamic therapy; photoimmunoconjugates.
Conflict of interest statement
Conflict of interest none
Figures








Similar articles
-
Optical Imaging, Photodynamic Therapy and Optically Triggered Combination Treatments.Cancer J. 2015 May-Jun;21(3):194-205. doi: 10.1097/PPO.0000000000000117. Cancer J. 2015. PMID: 26049699 Free PMC article. Review.
-
Chemo-photodynamic therapy with light-triggered disassembly of theranostic nanoplatform in combination with checkpoint blockade for immunotherapy of hepatocellular carcinoma.J Nanobiotechnology. 2021 Oct 30;19(1):355. doi: 10.1186/s12951-021-01101-1. J Nanobiotechnology. 2021. PMID: 34717654 Free PMC article.
-
Recent Advances in Photosensitizers as Multifunctional Theranostic Agents for Imaging-Guided Photodynamic Therapy of Cancer.Theranostics. 2021 Aug 26;11(18):9054-9088. doi: 10.7150/thno.62479. eCollection 2021. Theranostics. 2021. PMID: 34522227 Free PMC article. Review.
-
Supramolecular micelles as multifunctional theranostic agents for synergistic photodynamic therapy and hypoxia-activated chemotherapy.Acta Biomater. 2021 Sep 1;131:483-492. doi: 10.1016/j.actbio.2021.07.014. Epub 2021 Jul 13. Acta Biomater. 2021. PMID: 34265471
-
Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy.Biomedicines. 2021 Jan 16;9(1):85. doi: 10.3390/biomedicines9010085. Biomedicines. 2021. PMID: 33467201 Free PMC article. Review.
Cited by
-
Recent advances in noble metal complex based photodynamic therapy.Chem Sci. 2022 Apr 1;13(18):5085-5106. doi: 10.1039/d1sc05478c. eCollection 2022 May 11. Chem Sci. 2022. PMID: 35655575 Free PMC article. Review.
-
Photodynamic Priming Improves the Anti-Migratory Activity of Prostaglandin E Receptor 4 Antagonist in Cancer Cells In Vitro.Cancers (Basel). 2021 Oct 20;13(21):5259. doi: 10.3390/cancers13215259. Cancers (Basel). 2021. PMID: 34771424 Free PMC article.
-
Photodynamic priming modulates cellular ATP levels to overcome P-glycoprotein-mediated drug efflux in chemoresistant triple-negative breast cancer.Photochem Photobiol. 2025 Jan-Feb;101(1):188-205. doi: 10.1111/php.13970. Epub 2024 Jun 2. Photochem Photobiol. 2025. PMID: 38824410 Free PMC article.
-
Riboflavin-Mediated Photodynamic Therapy in Periodontology: A Systematic Review of Applications and Outcomes.Pharmaceutics. 2025 Feb 7;17(2):217. doi: 10.3390/pharmaceutics17020217. Pharmaceutics. 2025. PMID: 40006584 Free PMC article. Review.
-
Dual-Function Antibody Conjugate-Enabled Photoimmunotherapy Complements Fluorescence and Photoacoustic Imaging of Head and Neck Cancer Spheroids.Bioconjug Chem. 2024 Jan 17;35(1):51-63. doi: 10.1021/acs.bioconjchem.3c00406. Epub 2023 Dec 21. Bioconjug Chem. 2024. PMID: 38128912 Free PMC article.
References
-
- Photodiagn Huang Z.. Photodyn. Ther. 2008; 5: 285–287. - PubMed
-
- Blanco Kate C, Inada Natalia M, Salvio Ana Gabriela, Vollet-Filho José Dirceu and VS Bagnato. J. Tumor 2016; 4: 386–392.
-
- Frochot C and Mordon S. J. Porphyrins Phthalocyanines 2019; 23: 347–357.
Grants and funding
LinkOut - more resources
Full Text Sources
