Assessment of interleukin-10 promoter variant (-1082A/G) and cytokine production in patients with hemolytic uremic syndrome
- PMID: 37425258
- PMCID: PMC10327435
- DOI: 10.3389/fped.2023.1210158
Assessment of interleukin-10 promoter variant (-1082A/G) and cytokine production in patients with hemolytic uremic syndrome
Abstract
Introduction: Hemolytic uremic syndrome (HUS) is a condition that results in acute kidney failure mainly in children, which is caused by Shiga toxin-producing Escherichia coli and inflammatory response. Although anti-inflammatory mechanisms are triggered, studies on the implication in HUS are scarce. Interleukin-10 (IL-10) regulates inflammation in vivo, and the interindividual differences in its expression are related to genetic variants. Notably, the single nucleotide polymorphism (SNP) rs1800896 -1082 (A/G), located in the IL-10 promoter, regulates cytokine expression.
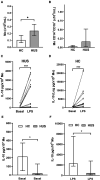
Methods: Plasma and peripheral blood mononuclear cells (PBMC) were collected from healthy children and HUS patients exhibiting hemolytic anemia, thrombocytopenia, and kidney damage. Monocytes identified as CD14+ cells were analyzed within PBMC by flow cytometry. IL-10 levels were quantified by ELISA, and SNP -1082 (A/G) was analyzed by allele-specific PCR.
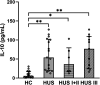
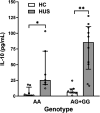
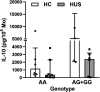
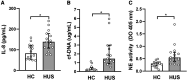
Results: Circulating IL-10 levels were increased in HUS patients, but PBMC from these patients exhibited a lower capacity to secrete this cytokine compared with those from healthy children. Interestingly, there was a negative association between the circulating levels of IL-10 and inflammatory cytokine IL-8. We observed that circulating IL-10 levels were threefold higher in HUS patients with -1082G allele in comparison to AA genotype. Moreover, there was relative enrichment of GG/AG genotypes in HUS patients with severe kidney failure.
Discussion: Our results suggest a possible contribution of SNP -1082 (A/G) to the severity of kidney failure in HUS patients that should be further evaluated in a larger cohort.
Keywords: HUS; IL-10; SNP; monocytes; renal failure.
© 2023 Mongelos, Sosa, Pineda, Fiorentino, Santiago, Abelleyro, Rossetti, Exeni, De Brasi, Palermo and Ramos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Exeni R. Síndrome urémico hemolítico. Archivos Latinoamericanos de Nefrología Pediátrica. (2001) 1:35–6. ISSN: 1667-4170
-
- Marta Adragna AB. Nefrologia pediatrica. 3rd ed. Buenos Aires: Sociedad Argentina de Pediatria; (2017).
LinkOut - more resources
Full Text Sources
Research Materials

