On the Selective Enzymatic Recycling of Poly(pentamethylene 2,5-furanoate)/Poly(lactic acid) Blends and Multiblock Copolymers
- PMID: 37425282
- PMCID: PMC10324456
- DOI: 10.1021/acssuschemeng.3c01796
On the Selective Enzymatic Recycling of Poly(pentamethylene 2,5-furanoate)/Poly(lactic acid) Blends and Multiblock Copolymers
Abstract
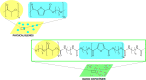
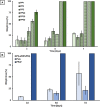
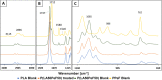
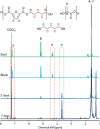
Among novel renewable furanoate-based polyesters, poly(pentamethylene 2,5-furandicarboxylate) (PPeF) shows outstanding gas barrier properties and high flexibility. PPeF blending/copolymerization with another well-known bio-based polymer, poly(lactic acid) (PLA), leads to considerably better mechanical and gas barrier properties of the latter, making it suitable for flexible food packaging applications. In this work, enzymatic depolymerization of PLA/PPeF blends with different compositions (1, 3, 5, 20, 30, and 50 wt % PPeF) and a PLA-PPeF block copolymer (50 wt % PPeF) by cutinase 1 from Thermobifida cellulositilytica (Thc_Cut1) was investigated as a possible recycling strategy. Based on quantification of weight loss and high-performance liquid chromatography (HPLC) analysis of released molecules, faster hydrolysis was seen for PLA/PPeF blends with increasing PPeF content when compared to neat PLA, while the block copolymer (P(LA50PeF50)) was significantly less susceptible to hydrolysis. Surface morphology analysis (via scanning electron microscopy), Fourier transform infrared spectroscopy, and NMR analysis confirmed preferential hydrolysis of the PPeF component. Through crystallization, 2,5-furandicarboxylic acid was selectively recovered from the depolymerized films and used for the resynthesis of the PPeF homopolymer, demonstrating the potential of enzymes for novel recycling concepts. The possibility of selective recovery of 2,5-furandicarboxylic acid from the completely depolymerized films with a 75% yield could bring further evidence of the high value of these materials, both in the form of blends and copolymers, for a sustainable whole packaging life cycle, where PPeF is potentially enzymatically recycled and PLA is mechanically recycled.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Generation of plastic packaging waste per capita (Eurostat). https://ec.europa.eu/eurostat/databrowser/view/cei_pc050/default/table?l... (accessed May 08, 2023).
-
- EU restrictions on certain single-use plastics. https://environment.ec.europa.eu/topics/plastics/single-use-plastics/eu-... (accessed May 08, 2023).
-
- European bioplastics. Fact sheet. What are bioplastics. European bioplastics, 2022https://docs.european-bioplastics.org/publications/fs/EuBP_FS_What_are_b... (accessed May 08, 2023).
LinkOut - more resources
Full Text Sources
