Concordance between cryobiopsy and forceps biopsy specimens in assessment of immunohistochemistry staining for non-small cell lung carcinoma
- PMID: 37425419
- PMCID: PMC10326773
- DOI: 10.21037/tlcr-22-621
Concordance between cryobiopsy and forceps biopsy specimens in assessment of immunohistochemistry staining for non-small cell lung carcinoma
Abstract
Background: Cryobiopsy is recently being promoted for biopsy of tumors in the lung periphery in precision medicine for lung cancer; the obtained tissue samples have been reported to be more useful compared to those obtained using forceps, because of the larger volume and higher quality. However, the influence of freezing and thawing of tissues when performing cryobiopsy on the results of immunohistochemistry (IHC) has not been completely understood.
Methods: In this study, consecutive patients who underwent diagnostic bronchoscopy with cryobiopsy for peripheral pulmonary lesions (PPLs) at our institution between June 2017 and November 2021 were reviewed retrospectively. Specimens of diagnosed cases of unresectable or recurrent non-small cell lung carcinoma (NSCLC) were selected. We compared the results of IHC assessment for programmed death-ligand 1 (PD-L1), human epidermal growth factor receptor 2 (HER2), and human epidermal growth factor receptor 3 (HER3) in cryobiopsy specimens versus conventional forceps biopsy specimens from the same site in the same procedure.
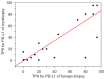
Results: Twenty-four of 40 patients were male (60%). The most frequent histologic type of cancer was adenocarcinoma (n=31, 77.5%), followed by NSCLC (n=4, 10%), squamous cell carcinoma (n=3, 7.5%), and others (n=2, 5%). The concordance rates of the tumor proportion scores (TPSs) for PD-L1, IHC score for HER2 and, IHC scores for HER3 were 85%, 72.5%, and 75%, respectively; the weighted kappa were 0.835, 0.637, and 0.697, respectively.
Conclusions: Freezing and thawing associated with cryobiopsy had virtually no effect on the results of IHC. We suggest that cryobiopsy specimens would therefore be ideal for precision medicine and translational research.
Keywords: Cryobiopsy; human epidermal growth factor receptor 2 (HER2); human epidermal growth factor receptor 3 (HER3); immunohistochemistry (IHC); lung cancer.
2023 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-621/coif). YO serves as an unpaid editorial board member of Translational Lung Cancer Research from January 2020 to December 2023. YM reports research fund from Hitachi and honoraria from Olympus, AstraZeneca, NOVARTIS, COOK, AMCO, Thermo Fisher Scientific, Erbe Elektromedizin GmbH, Fujifilm, Chugai, Eli Lilly, Merck and Takeda. TI reports grants from Hitachi High-Tech Corporation and honoraria from COOK, Chugai pharma, Eli Lilly, Olympus, Novartis pharma, Fujifilm and Thermo Fisher Scientific K.K. KU reports grants from Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Nos. JP22K15698 JP19K16966), and receives payments for lectures from Novartis, Thermo Fisher Scientific, AstraZeneca and Chugai. TY reports lecture fees from AstraZeneca, Chugai, Ono, Eli Lilly, and Takeda, and research funds from AMGEN, AstraZeneca, Takeda, Daiichi-Sankyo, Ono, MSD, AbbVie, Novartis, Chugai, Novartis, Chugai, Merck, Blueprint, and BMS. YG reports grants from AZK, Pfizer, Abbvie, Eli Lilly, Pfizer, Bristol Myers Squibb, Ono, Novartis, Kyorin, DaiichiSankyo, Novartis, and Prefered Networ, honoraria from Eli Lilly, Chugai,Taiho, Boehringer Ingelheim, Ono, Bristol Myers Squibb, Pfizer, MSD, Novartis. Merck, and Thermo Fischer, participation on an advisory board from AstraZeneca, Chugai, Boehringer Ingelheim, Eli Lilly, Taiho, Pfizer, Novartis, Guardant Health Inc., Illumina, DaiichiSankyo, Ono Pharmaceutical, Bristol Myers Squibb, and MSD, and leadership in Cancer Net Japan, JAMT. HH serves as a consultant to AstraZeneca, Eli Lilly, Chugai, Roche, Ono, BMS, and MSD, and receives lecture fees from AstraZeneca, MSD, Eli Lilly Ono, BMS, Chugai, Roche, Kyowa-Kirin, and Novartis, and research funds from MSD, AstraZeneca, BMS, Ono, Merck Biopharma, Daiichi-Sankyo, Janssen, Genomic Health, Chugai, Roche, and Novartis. Yuichiro Ohe receives lecture fees from AstraZeneca and Chugai, and research funds from AstraZeneca, Chugai, Eli Lilly, Ono, BMS, Kyorin, Dainippon-Sumitomo, Pfizer, Taiho, Novartis, Takeda, Kissei, Daiichi-Sankyo, Janssen, and LOXO. YY reports honoraria for lectures from MDS, Chugai-pharma, AstraZeneca, Novartis, Pfizer, Thermo Fisher Science, and Amgen. The other authors have no conflicts of interest to declare.
Figures


Similar articles
-
Feasibility study of cryobiopsy for practical pathological diagnosis of primary lung cancer including immunohistochemical assessment.Jpn J Clin Oncol. 2021 Feb 8;51(2):271-278. doi: 10.1093/jjco/hyaa174. Jpn J Clin Oncol. 2021. PMID: 32964232
-
Cryobiopsy increases the EGFR detection rate in non-small cell lung cancer.Lung Cancer. 2020 Mar;141:56-63. doi: 10.1016/j.lungcan.2019.12.008. Epub 2020 Jan 7. Lung Cancer. 2020. PMID: 31955001 Clinical Trial.
-
Comparative analysis of programmed death ligand 1 expression in paired cytologic and histologic specimens of non-small cell lung cancer.Cancer Cytopathol. 2020 Aug;128(8):580-588. doi: 10.1002/cncy.22292. Epub 2020 May 28. Cancer Cytopathol. 2020. PMID: 32463583
-
Efficacy and Safety of Cryobiopsy vs. Forceps Biopsy for Interstitial Lung Diseases, Lung Tumors, and Peripheral Pulmonary Lesions: An Updated Systematic Review and Meta-Analysis.Front Med (Lausanne). 2022 Mar 10;9:840702. doi: 10.3389/fmed.2022.840702. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35372452 Free PMC article.
-
Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non-Small-Cell Lung Cancer.J Clin Oncol. 2017 Dec 1;35(34):3867-3876. doi: 10.1200/JCO.2017.74.7642. Epub 2017 Oct 20. J Clin Oncol. 2017. PMID: 29053400 Review.
Cited by
-
Diagnosis of Peripheral Pulmonary Lesions Using Forceps and 1.1- or 1.7-mm Cryoprobes: A Randomised Trial.Respiration. 2025;104(8):603-614. doi: 10.1159/000545338. Epub 2025 Mar 25. Respiration. 2025. PMID: 40132574 Free PMC article. Clinical Trial.
References
-
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. 10.1016/S1470-2045(09)70364-X - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
