Reusable nano-catalyzed green protocols for the synthesis of quinoxalines: an overview
- PMID: 37425629
- PMCID: PMC10326672
- DOI: 10.1039/d3ra03646d
Reusable nano-catalyzed green protocols for the synthesis of quinoxalines: an overview
Abstract
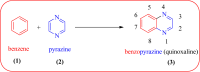
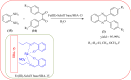
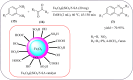
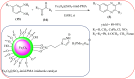
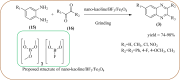
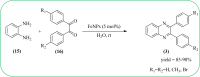
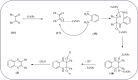
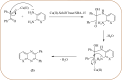
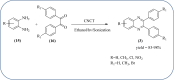
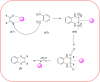
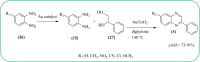
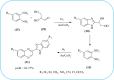
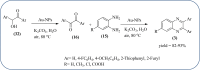
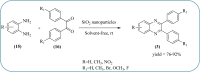
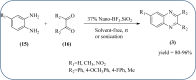
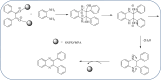
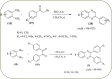
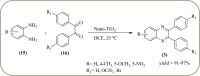
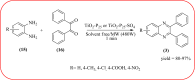
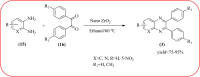
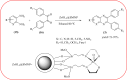
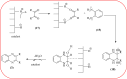
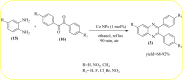
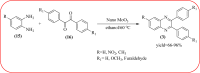
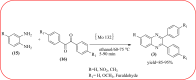
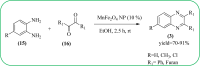
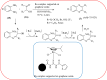
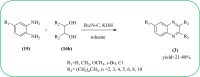
Heterocyclic compounds are very widely distributed in nature and are essential for life activities. They play a vital role in the metabolism of all living cells, for example, vitamins and co-enzyme precursors thiamine, riboflavin etc. Quinoxalines are a class of N-heterocycles that are present in a variety of natural and synthetic compounds. The distinct pharmacological activities of quinoxalines have attracted medicinal chemists considerably over the past few decades. Quinoxaline-based compounds possess extensive potential applications as medicinal drugs, presently; more than fifteen drugs are available for the treatment of different diseases. Diverse synthetic protocols have been developed via a one-pot approach using efficient catalysts, reagents, and nano-composites/nanocatalysts etc. But the use of homogeneous and transition metal-based catalysts suffers some demerits such as low atom economy, recovery of catalysts, harsh reaction conditions, extended reaction period, expensive catalysts, the formation of by-products, and unsatisfactory yield of products as well as toxic solvents. These drawbacks have shifted the attention of chemists/researchers to develop green and efficient protocols for synthesizing quinoxaline derivatives. In this context, many efficient methods have been developed for the synthesis of quinoxalines using nanocatalysts or nanostructures. In this review, we have summarized the recent progress (till 2023) in the nano-catalyzed synthesis of quinoxalines using condensation of o-phenylenediamine with diketone/other reagents with plausible mechanistic details. With this review, we hope that some more efficient ways of synthesizing quinoxalines can be developed by synthetic chemists.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declared that they have no conflict of interest.
Figures

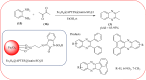
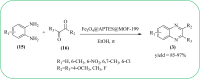
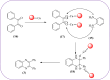
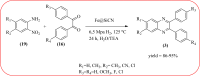











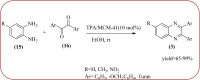




Similar articles
-
Quinoline Synthesis: Nanocatalyzed Green Protocols-An Overview.ACS Omega. 2024 Oct 14;9(42):42630-42667. doi: 10.1021/acsomega.4c07011. eCollection 2024 Oct 22. ACS Omega. 2024. PMID: 39464456 Free PMC article. Review.
-
An insight into medicinal chemistry of anticancer quinoxalines.Bioorg Med Chem. 2019 Jan 1;27(1):16-35. doi: 10.1016/j.bmc.2018.11.021. Epub 2018 Nov 15. Bioorg Med Chem. 2019. PMID: 30502116 Review.
-
Recent advances in the transition-metal-free synthesis of quinoxalines.RSC Adv. 2021 Nov 19;11(59):37325-37353. doi: 10.1039/d1ra06942j. eCollection 2021 Nov 17. RSC Adv. 2021. PMID: 35496411 Free PMC article. Review.
-
A green and efficient protocol for the synthesis of quinoxaline, benzoxazole and benzimidazole derivatives using heteropolyanion-based ionic liquids: as a recyclable solid catalyst.Comb Chem High Throughput Screen. 2013 Sep;16(8):618-27. doi: 10.2174/1386207311316080004. Comb Chem High Throughput Screen. 2013. PMID: 23547570
-
Multicomponent Synthesis Strategies, Catalytic Activities, and Potential Therapeutic Applications of Pyranocoumarins: A Comprehensive Review.Chem Biodivers. 2023 Oct;20(10):e202300836. doi: 10.1002/cbdv.202300836. Epub 2023 Oct 5. Chem Biodivers. 2023. PMID: 37702294 Review.
Cited by
-
Quinoline Synthesis: Nanocatalyzed Green Protocols-An Overview.ACS Omega. 2024 Oct 14;9(42):42630-42667. doi: 10.1021/acsomega.4c07011. eCollection 2024 Oct 22. ACS Omega. 2024. PMID: 39464456 Free PMC article. Review.
-
Effects of dietary chito-oligosaccharide and β-glucan on the water quality and gut microbiota, intestinal morphology, immune response, and meat quality of Chinese soft-shell turtle (Pelodiscus sinensis).Front Immunol. 2023 Oct 26;14:1266997. doi: 10.3389/fimmu.2023.1266997. eCollection 2023. Front Immunol. 2023. PMID: 38022669 Free PMC article.
References
-
- Pozharskii A. F., Toldatenkov A. and Katritzky A. R., Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry and Applications, Wiley publication, 2011, 2nd edn
-
- Ameta K. L., Kant R., Penoni A., Maspero A. and Scapinello L., N-Heterocycles Synthesis and Biological Evaluation, Springer, Singapore, 2022
-
- Narasimha Reddy Y. Reddy Mardi R. Reddy G. N. Reddy T. S. Seku K. Fahmy H. M. Abdel-Hafez S. H. Hessien M. M. Shalan A. E. J. Mol. Struct. 2022;1253:132260.
-
- Xu K. Wang X. Cheng L. Cui Q. Shi J. Zhang L. Chen S. Bioorg. Med. Chem. 2023;78(15):117152. - PubMed
-
- Keri R. S. Pandule S. S. Budagumpi S. Nagaraja B. M. Arch. Pharm. 2018;351:e1700325. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

