This is a preprint.
Dysregulated Pulmonary Inflammatory Responses Exacerbate the Outcome of Secondary Aspergillosis Following Influenza
- PMID: 37425745
- PMCID: PMC10326983
- DOI: 10.1101/2023.06.27.546808
Dysregulated Pulmonary Inflammatory Responses Exacerbate the Outcome of Secondary Aspergillosis Following Influenza
Update in
-
Dysregulated pulmonary inflammatory responses exacerbate the outcome of secondary aspergillosis following influenza.mBio. 2023 Oct 31;14(5):e0163323. doi: 10.1128/mbio.01633-23. Epub 2023 Sep 8. mBio. 2023. PMID: 37681974 Free PMC article.
Abstract
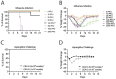
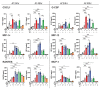
Inhalation of airborne conidia of the ubiquitous fungus Aspergillus fumigatus commonly occurs but invasive aspergillosis is rare except in profoundly immunocompromised persons. Severe influenza predisposes patients to invasive pulmonary aspergillosis by mechanisms that are poorly defined. Using a post-influenza aspergillosis model, we found that superinfected mice had 100% mortality when challenged with A. fumigatus conidia on days 2 and 5 (early stages) of influenza A virus infection but 100% survival when challenged on days 8 and 14 (late stages). Influenza-infected mice superinfected with A. fumigatus had increased levels of the pro-inflammatory cytokines and chemokines IL-6, TNFα, IFNβ, IL-12p70, IL-1α, IL-1β, CXCL1, G-CSF, MIP-1α, MIP-1β, RANTES and MCP-1. Surprisingly, on histopathological analysis, superinfected mice did not have greater lung inflammation compared with mice infected with influenza alone. Mice infected with influenza had dampened neutrophil recruitment to the lungs following subsequent challenge with A. fumigatus , but only if the fungal challenge was executed during the early stages of influenza infection. However, influenza infection did not have a major effect on neutrophil phagocytosis and killing of A. fumigatus conidia. Moreover, minimal germination of conidia was seen on histopathology even in the superinfected mice. Taken together, our data suggest that the high mortality rate seen in mice during the early stages of influenza-associated pulmonary aspergillosis is multifactorial, with a greater contribution from dysregulated inflammation than microbial growth.
Importance: Severe influenza is a risk factor for fatal invasive pulmonary aspergillosis; however, the mechanistic basis for the lethality is unclear. Utilizing an influenza-associated pulmonary aspergillosis (IAPA) model, we found that mice infected with influenza A virus followed by A. fumigatus had 100% mortality when superinfected during the early stages of influenza but survived at later stages. While superinfected mice had dysregulated pulmonary inflammatory responses compared to controls, they had neither increased inflammation nor extensive fungal growth. Although influenza-infected mice had dampened neutrophil recruitment to the lungs following subsequent challenge with A. fumigatus , influenza did not affect the ability of neutrophils to clear the fungi. Our data suggest that the lethality seen in our model IAPA is multifactorial with dysregulated inflammation being a greater contributor than uncontrollable microbial growth. If confirmed in humans, our findings provide a rationale for clinical studies of adjuvant anti-inflammatory agents in the treatment of IAPA.
Figures






Similar articles
-
Dysregulated pulmonary inflammatory responses exacerbate the outcome of secondary aspergillosis following influenza.mBio. 2023 Oct 31;14(5):e0163323. doi: 10.1128/mbio.01633-23. Epub 2023 Sep 8. mBio. 2023. PMID: 37681974 Free PMC article.
-
Postinfluenza Environment Reduces Aspergillus fumigatus Conidium Clearance and Facilitates Invasive Aspergillosis In Vivo.mBio. 2022 Dec 20;13(6):e0285422. doi: 10.1128/mbio.02854-22. Epub 2022 Nov 15. mBio. 2022. PMID: 36377895 Free PMC article.
-
Influenza Suppresses Neutrophil Recruitment to the Lung and Exacerbates Secondary Invasive Pulmonary Aspergillosis.J Immunol. 2020 Jul 15;205(2):480-488. doi: 10.4049/jimmunol.2000067. Epub 2020 Jun 10. J Immunol. 2020. PMID: 32522833 Free PMC article.
-
Pulmonary defense mechanisms against opportunistic fungal pathogens.Immunol Ser. 1989;47:243-71. Immunol Ser. 1989. PMID: 2490078 Review.
-
The innate immune response to Aspergillus fumigatus at the alveolar surface.FEMS Microbiol Rev. 2015 Sep;39(5):670-87. doi: 10.1093/femsre/fuv018. Epub 2015 Apr 30. FEMS Microbiol Rev. 2015. PMID: 25934117 Review.
References
-
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv13. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
