This is a preprint.
Focusing antibody responses to the fusion peptide in rhesus macaques
- PMID: 37425865
- PMCID: PMC10327030
- DOI: 10.1101/2023.06.26.545779
Focusing antibody responses to the fusion peptide in rhesus macaques
Update in
-
Priming antibody responses to the fusion peptide in rhesus macaques.NPJ Vaccines. 2024 Jul 12;9(1):126. doi: 10.1038/s41541-024-00918-9. NPJ Vaccines. 2024. PMID: 38997302 Free PMC article.
Abstract
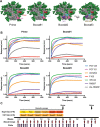
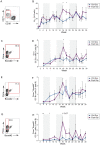
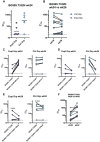
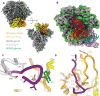
Immunodominance of antibodies targeting non-neutralizing epitopes and the high level of somatic hypermutation within germinal centers (GCs) required for most HIV broadly neutralizing antibodies (bnAbs) are major impediments to the development of an effective HIV vaccine. Rational protein vaccine design and non-conventional immunization strategies are potential avenues to overcome these hurdles. Here, we report using implantable osmotic pumps to continuously deliver a series of epitope-targeted immunogens to rhesus macaques over the course of six months to elicit immune responses against the conserved fusion peptide. Antibody specificities and GC responses were tracked longitudinally using electron microscopy polyclonal epitope mapping (EMPEM) and lymph node fine-needle aspirates, respectively. Application of cryoEMPEM delineated key residues for on-target and off-target responses that can drive the next round of structure-based vaccine design.
Conflict of interest statement
Conflicts of Interest None.
Figures

Similar articles
-
Priming antibody responses to the fusion peptide in rhesus macaques.NPJ Vaccines. 2024 Jul 12;9(1):126. doi: 10.1038/s41541-024-00918-9. NPJ Vaccines. 2024. PMID: 38997302 Free PMC article.
-
Slow Delivery Immunization Enhances HIV Neutralizing Antibody and Germinal Center Responses via Modulation of Immunodominance.Cell. 2019 May 16;177(5):1153-1171.e28. doi: 10.1016/j.cell.2019.04.012. Epub 2019 May 9. Cell. 2019. PMID: 31080066 Free PMC article.
-
Vaccine induction of CD4-mimicking HIV-1 broadly neutralizing antibody precursors in macaques.Cell. 2024 Jan 4;187(1):79-94.e24. doi: 10.1016/j.cell.2023.12.002. Cell. 2024. PMID: 38181743 Free PMC article.
-
Structure-guided envelope trimer design in HIV-1 vaccine development: a narrative review.J Int AIDS Soc. 2021 Nov;24 Suppl 7(Suppl 7):e25797. doi: 10.1002/jia2.25797. J Int AIDS Soc. 2021. PMID: 34806305 Free PMC article. Review.
-
Strategies for induction of HIV-1 envelope-reactive broadly neutralizing antibodies.J Int AIDS Soc. 2021 Nov;24 Suppl 7(Suppl 7):e25831. doi: 10.1002/jia2.25831. J Int AIDS Soc. 2021. PMID: 34806332 Free PMC article. Review.
References
-
- National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(2):1–64. - PubMed
-
- Watson JC, Pearson JA, Markowitz LE, et al. An evaluation of measles revaccination among school-entry-aged children. Pediatrics. 1996;97(5):613–618. - PubMed
-
- Poland GA, Jacobson RM, Thampy AM, et al. Measles reimmunization in children seronegative after initial immunization. JAMA. 1997;277(14):1156–1158. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
