This is a preprint.
Increased circulating fibronectin, depletion of natural IgM and heightened EBV, HSV-1 reactivation in ME/CFS and long COVID
- PMID: 37425897
- PMCID: PMC10327231
- DOI: 10.1101/2023.06.23.23291827
Increased circulating fibronectin, depletion of natural IgM and heightened EBV, HSV-1 reactivation in ME/CFS and long COVID
Abstract
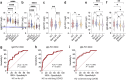
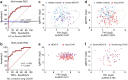
Myalgic Encephalomyelitis/ Chronic Fatigue syndrome (ME/CFS) is a complex, debilitating, long-term illness without a diagnostic biomarker. ME/CFS patients share overlapping symptoms with long COVID patients, an observation which has strengthened the infectious origin hypothesis of ME/CFS. However, the exact sequence of events leading to disease development is largely unknown for both clinical conditions. Here we show antibody response to herpesvirus dUTPases, particularly to that of Epstein-Barr virus (EBV) and HSV-1, increased circulating fibronectin (FN1) levels in serum and depletion of natural IgM against fibronectin ((n)IgM-FN1) are common factors for both severe ME/CFS and long COVID. We provide evidence for herpesvirus dUTPases-mediated alterations in host cell cytoskeleton, mitochondrial dysfunction and OXPHOS. Our data show altered active immune complexes, immunoglobulin-mediated mitochondrial fragmentation as well as adaptive IgM production in ME/CFS patients. Our findings provide mechanistic insight into both ME/CFS and long COVID development. Finding of increased circulating FN1 and depletion of (n)IgM-FN1 as a biomarker for the severity of both ME/CFS and long COVID has an immediate implication in diagnostics and development of treatment modalities.
Keywords: (n)IgM; EBV; Fibronectin; HHV-6; HSV-1; ME/CFS; dUTPase; long COVID.
Conflict of interest statement
Competing Interests: The authors declare that they have no competing interests.
Figures
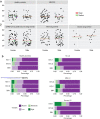
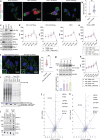






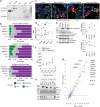
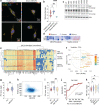
References
References for Methods:
-
- Horn A. et al. Long-term health sequelae and quality of life at least 6 months after infection with SARS-CoV-2: design and rationale of the COVIDOM-study as part of the NAPKON population-based cohort platform (POP). Infection 49, 1277–1287, doi: 10.1007/s15010-021-01707-5 (2021). - DOI - PMC - PubMed
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
