This is a preprint.
SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy
- PMID: 37425934
- PMCID: PMC10327262
- DOI: 10.1101/2023.06.23.23288598
SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy
Update in
-
SARS-CoV-2 Virologic Rebound With Nirmatrelvir-Ritonavir Therapy : An Observational Study.Ann Intern Med. 2023 Dec;176(12):1577-1585. doi: 10.7326/M23-1756. Epub 2023 Nov 14. Ann Intern Med. 2023. PMID: 37956428 Free PMC article.
Abstract
Objective: To compare the frequency of replication-competent virologic rebound with and without nirmatrelvir-ritonavir treatment for acute COVID-19. Secondary aims were to estimate the validity of symptoms to detect rebound and the incidence of emergent nirmatrelvir-resistance mutations after rebound.
Design: Observational cohort study.
Setting: Multicenter healthcare system in Boston, Massachusetts.
Participants: We enrolled ambulatory adults with a positive COVID-19 test and/or a prescription for nirmatrelvir-ritonavir.
Exposures: Receipt of 5 days of nirmatrelvir-ritonavir treatment versus no COVID-19 therapy.
Main outcome and measures: The primary outcome was COVID-19 virologic rebound, defined as either (1) a positive SARS-CoV-2 viral culture following a prior negative culture or (2) two consecutive viral loads ≥4.0 log10 copies/milliliter after a prior reduction in viral load to <4.0 log10 copies/milliliter.
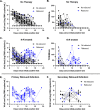
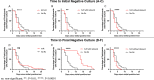
Results: Compared with untreated individuals (n=55), those taking nirmatrelvir-ritonavir (n=72) were older, received more COVID-19 vaccinations, and were more commonly immunosuppressed. Fifteen individuals (20.8%) taking nirmatrelvir-ritonavir experienced virologic rebound versus one (1.8%) of the untreated (absolute difference 19.0% [95%CI 9.0-29.0%], P=0.001). In multivariable models, only N-R was associated with VR (AOR 10.02, 95%CI 1.13-88.74). VR occurred more commonly among those with earlier nirmatrelvir-ritonavir initiation (29.0%, 16.7% and 0% when initiated days 0, 1, and ≥2 after diagnosis, respectively, P=0.089). Among participants on N-R, those experiencing rebound had prolonged shedding of replication-competent virus compared to those that did not rebound (median: 14 vs 3 days). Only 8/16 with virologic rebound reported worsening symptoms (50%, 95%CI 25%-75%); 2 were completely asymptomatic. We detected no post-rebound nirmatrelvir-resistance mutations in the NSP5 protease gene.
Conclusions and relevance: Virologic rebound occurred in approximately one in five people taking nirmatrelvir-ritonavir and often occurred without worsening symptoms. Because it is associated with replication-competent viral shedding, close monitoring and potential isolation of those who rebound should be considered.
Figures

Similar articles
-
SARS-CoV-2 Virologic Rebound With Nirmatrelvir-Ritonavir Therapy : An Observational Study.Ann Intern Med. 2023 Dec;176(12):1577-1585. doi: 10.7326/M23-1756. Epub 2023 Nov 14. Ann Intern Med. 2023. PMID: 37956428 Free PMC article.
-
COVID-19 Rebound After VV116 vs Nirmatrelvir-Ritonavir Treatment: A Randomized Clinical Trial.JAMA Netw Open. 2024 Mar 4;7(3):e241765. doi: 10.1001/jamanetworkopen.2024.1765. JAMA Netw Open. 2024. PMID: 38477921 Free PMC article. Clinical Trial.
-
Emerging SARS-CoV-2 Resistance After Antiviral Treatment.JAMA Netw Open. 2024 Sep 3;7(9):e2435431. doi: 10.1001/jamanetworkopen.2024.35431. JAMA Netw Open. 2024. PMID: 39320890 Free PMC article.
-
Coronavirus disease 2019 rebounds following nirmatrelvir/ritonavir treatment.J Med Virol. 2023 Feb;95(2):e28430. doi: 10.1002/jmv.28430. J Med Virol. 2023. PMID: 36571273 Free PMC article. Review.
-
Paxlovid (Nirmatrelvir/Ritonavir): A new approach to Covid-19 therapy?Biomed Pharmacother. 2023 Jun;162:114367. doi: 10.1016/j.biopha.2023.114367. Epub 2023 Feb 6. Biomed Pharmacother. 2023. PMID: 37018987 Free PMC article. Review.
References
-
- Pandit JA, Radin JM, Chiang D, et al. The COVID-19 Rebound Study: A Prospective Cohort Study to Evaluate Viral and Symptom Rebound Differences in Participants Treated with Nirmatrelvir Plus Ritonavir Versus Untreated Controls. Clin Infect Dis. Published online February 22, 2023:ciad102. doi:10.1093/cid/ciad102 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
