This is a preprint.
Visualizing alpha-synuclein and iron deposition in M83 mouse model of Parkinson's disease in vivo
- PMID: 37425954
- PMCID: PMC10327184
- DOI: 10.1101/2023.06.28.546962
Visualizing alpha-synuclein and iron deposition in M83 mouse model of Parkinson's disease in vivo
Update in
-
Visualizing alpha-synuclein and iron deposition in M83 mouse model of Parkinson's disease in vivo.Brain Pathol. 2024 Nov;34(6):e13288. doi: 10.1111/bpa.13288. Epub 2024 Jul 9. Brain Pathol. 2024. PMID: 38982662 Free PMC article.
Abstract
Background: Abnormal alpha-synuclein and iron accumulation in the brain play an important role in Parkinson's disease (PD). Herein, we aim at visualizing alpha-synuclein inclusions and iron deposition in the brains of M83 (A53T) mouse models of PD in vivo.
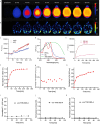
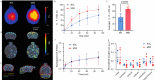
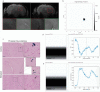
Methods: Fluorescently labelled pyrimidoindole-derivative THK-565 was characterized by using recombinant fibrils and brains from 10-11 months old M83 mice, which subsequently underwent in vivo concurrent wide-field fluorescence and volumetric multispectral optoacoustic tomography (vMSOT) imaging. The in vivo results were verified against structural and susceptibility weighted imaging (SWI) magnetic resonance imaging (MRI) at 9.4 Tesla and scanning transmission X-ray microscopy (STXM) of perfused brains. Brain slice immunofluorescence and Prussian blue staining were further performed to validate the detection of alpha-synuclein inclusions and iron deposition in the brain, respectively.
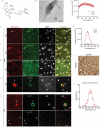
Results: THK-565 showed increased fluorescence upon binding to recombinant alpha-synuclein fibrils and alpha-synuclein inclusions in post-mortem brain slices from patients with Parkinson's disease and M83 mice. i.v. administration of THK-565 in M83 mice showed higher cerebral retention at 20 and 40 minutes post-injection by wide-field fluorescence compared to non-transgenic littermate mice, in congruence with the vMSOT findings. SWI/phase images and Prussian blue indicated the accumulation of iron deposits in the brains of M83 mice, presumably in the Fe3+ form, as evinced by the STXM results.
Conclusion: We demonstrated in vivo mapping of alpha-synuclein by means of non-invasive epifluorescence and vMSOT imaging assisted with a targeted THK-565 label and SWI/STXM identification of iron deposits in M83 mouse brains ex vivo.
Keywords: Parkinson’s disease; alpha-synuclein; fluorescence imaging; iron; magnetic resonance imaging; optoacoustic imaging; susceptibility weighted imaging.
Conflict of interest statement
Disclosures The authors declare no conflicts of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
