Immune protection induced by E2 recombinant glycoprotein of bovine viral diarrhea virus in a murine model
- PMID: 37426077
- PMCID: PMC10324609
- DOI: 10.3389/fvets.2023.1168846
Immune protection induced by E2 recombinant glycoprotein of bovine viral diarrhea virus in a murine model
Abstract
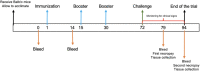
Bovine viral diarrhea virus (BVDV) is considered the most important viral pathogen in ruminants worldwide due to the broad range of clinical manifestations displayed by infected animals. Therefore, infection with BVDV leads to severe economic losses in several countries' beef and dairy industries. Vaccination prevents reproductive failure and gastrointestinal and respiratory disorders caused by BVDV infection. However, considering their limitations, conventional vaccines such as live, attenuated, and killed viruses have been applied. Hence, different studies have described subunit vaccines as an effective and safe alternative for BVDV protection. Therefore, in this study, the ectodomain of E2 (E2e) glycoprotein from NADL BVDV strain was expressed in mammalian cells and used in two vaccine formulations to evaluate immunogenicity and protection against BVDV conferred in a murine model. Formulations consisted of solo E2e glycoprotein and E2e glycoprotein emulsified in adjuvant ISA 61 VG. Five groups of 6 mice of 6-to-8-week-old were immunized thrice on days 1, 15, and 30 by intraperitoneal injection with the mentioned formulations and controls. To evaluate the conferred protection against BVDV, mice were challenged six weeks after the third immunization. In addition, the humoral immune response was evaluated after vaccination and challenge. Mice groups inoculated with solo E2e and the E2e + ISA 61 VG displayed neutralizing titers; however, the E2 antibody titers in the E2e + ISA 61 VG group were significantly higher than the mice group immunized with the solo E2e glycoprotein. In addition, immunization using E2e + ISA 61 VG prevents animals from developing severe lesions in surveyed tissues. Moreover, this group acquired protection against the BVDV challenge, evidenced by a significant reduction of positive staining for BVDV antigen in the lungs, liver, and brain between the experimental groups. Our findings demonstrated that using E2e + ISA 61 VG induces greater BVDV protection by an early humoral response and reduced histopathological lesions and BVDV antigen detection in affected organs, indicating that E2e + ISA 61 VG subunit formulation can be considered as a putative vaccine candidate against BVDV. The efficacy and safety of this vaccine candidate in cattle requires further investigation.
Keywords: E2 recombinant glycoprotein; adjuvant; bovine viral diarrhea virus; murine model; virus neutralization.
Copyright © 2023 Gómez-Romero, Arias, Verdugo-Rodríguez, López, Valenzuela-Moreno, Cedillo-Peláez and Basurto-Alcántara.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Host Immune Response to Bovine Viral Diarrhea Virus (BVDV): Insights and Strategies for Effective Vaccine Design.Vaccines (Basel). 2025 Apr 25;13(5):456. doi: 10.3390/vaccines13050456. Vaccines (Basel). 2025. PMID: 40432068 Free PMC article. Review.
-
Enhanced immune responses to E2 protein and DNA formulated with ISA 61 VG administered as a DNA prime-protein boost regimen against bovine viral diarrhea virus.Vaccine. 2018 Sep 5;36(37):5591-5599. doi: 10.1016/j.vaccine.2018.07.054. Epub 2018 Aug 1. Vaccine. 2018. PMID: 30077483
-
Dendritic Cell Targeting of Bovine Viral Diarrhea Virus E2 Protein Expressed by Lactobacillus casei Effectively Induces Antigen-Specific Immune Responses via Oral Vaccination.Viruses. 2019 Jun 25;11(6):575. doi: 10.3390/v11060575. Viruses. 2019. PMID: 31242608 Free PMC article.
-
Optimum processing conditions for a trivalent-inactivated bovine viral diarrhea virus (BVDV) vaccine using field strains and immunogenicity of candidate formulations with different adjuvants.Vet Res Commun. 2024 Nov 26;49(1):37. doi: 10.1007/s11259-024-10608-5. Vet Res Commun. 2024. PMID: 39589623
-
Maternal antibody blocks humoral but not T cell responses to BVDV.Biologicals. 2003 Jun;31(2):123-5. doi: 10.1016/s1045-1056(03)00027-7. Biologicals. 2003. PMID: 12770543 Review.
Cited by
-
Recombinant Subunit Vaccine Candidate against the Bovine Viral Diarrhea Virus.Int J Mol Sci. 2024 Aug 10;25(16):8734. doi: 10.3390/ijms25168734. Int J Mol Sci. 2024. PMID: 39201420 Free PMC article.
-
Host Immune Response to Bovine Viral Diarrhea Virus (BVDV): Insights and Strategies for Effective Vaccine Design.Vaccines (Basel). 2025 Apr 25;13(5):456. doi: 10.3390/vaccines13050456. Vaccines (Basel). 2025. PMID: 40432068 Free PMC article. Review.
-
Developing a PmSLP3-based vaccine formulation that provides robust long-lasting protection against hemorrhagic septicemia-causing serogroup B and E strains of Pasteurella multocida in cattle.Front Immunol. 2024 May 21;15:1392681. doi: 10.3389/fimmu.2024.1392681. eCollection 2024. Front Immunol. 2024. PMID: 38835751 Free PMC article.
References
-
- Orban S, Liess B, Hafez SM, Frey HR, Blindow H, Sasse-Patzer B, et al. . Studies on transplacental transmissibility of a Bovine Virus Diarrhoea (BVD) vaccine virus. I Inoculation of pregnant cows 15 to 90 days before parturition (190th to 265th day of gestation). Zentralbl Veterinarmed B. (1983) 30:619–34. 10.1111/j.1439-0450.1983.tb01888.x - DOI - PubMed
LinkOut - more resources
Full Text Sources

