NASAFYTOL® supplementation in adults hospitalized with COVID-19 infection: results from an exploratory open-label randomized controlled trial
- PMID: 37426178
- PMCID: PMC10324407
- DOI: 10.3389/fnut.2023.1137407
NASAFYTOL® supplementation in adults hospitalized with COVID-19 infection: results from an exploratory open-label randomized controlled trial
Abstract
Objectives: The effect and safety of Nasafytol®, a food supplement combining curcumin, quercetin, and Vitamin D, on hospitalized COVID-19-positive patients as support to standard of care were to be assessed.
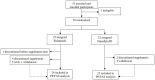
Methods: This exploratory, open-label, randomized, controlled trial was carried out among hospitalized adults with COVID-19 infection. Participants were randomly assigned to receive Nasafytol® or Fultium® control. The improvement of the clinical condition and occurrence of (serious) adverse events were evaluated. The study was registered on clincaltrials.gov with the identifier NCT04844658.
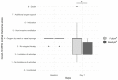
Results: Twenty-five patients received Nasafytol®, and 24 received Fultium®. Demographic characteristics were well balanced between the groups. On day 14 (or at hospital leave if < 14 days), no difference was observed between groups regarding their clinical condition, fever, or the need of oxygen therapy. At day 7, however, 19 participants had been discharged from the hospital in the Nasafytol® arm compared to 10 participants in the Fultium® arm. No participants were transferred to the ICU or died in the Nasafytol® arm, vs. 4 transfers and 1 death in the Fultium® arm. The clinical condition of participants in the Nasafytol® arm had improved, as evidenced by a decrease in the COVID-19 WHO score. Interestingly, five SAEs occurred with Fultium®, while no SAE was observed with Nasafytol®.
Conclusion: Supplementation with Nasafytol®, in addition to standard-of-care treatment, led to a faster discharge from the hospital, improved clinical conditions of participants, and a reduced risk of serious outcomes, including transfer to the intensive care unit or death, in patients hospitalized with COVID-19.
Keywords: COVID-19; SARS–CoV-2; clinical trial; coronavirus; curcumine; food supplement; quercetine; vitamin D.
Copyright © 2023 Gérain, Uebelhoer, Costes, Herman, Pietri, Donneau, Monseur and Henrotin.
Conflict of interest statement
YH is the founder and president of Artialis SA, a spin-off company of the University of Liège. YH has also received consulting and speaker fees from Tilman SA, Nestlé, Laboratoire Expanscience, Heel, Megalab, Genequine, LABHRA, and Biose. MU, BC, JH, and SP are employees of Artialis SA. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
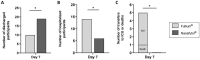
Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Management of Renin-Angiotensin-Aldosterone System blockade in patients admitted to hospital with confirmed coronavirus disease (COVID-19) infection (The McGill RAAS-COVID- 19): A structured summary of a study protocol for a randomized controlled trial.Trials. 2021 Feb 5;22(1):115. doi: 10.1186/s13063-021-05080-4. Trials. 2021. PMID: 33546734 Free PMC article.
-
Randomized clinical trial to evaluate safety and efficacy of convalescent plasma use among hospitalized patients with COVID-19 (PERUCONPLASMA): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 May 17;22(1):342. doi: 10.1186/s13063-021-05189-6. Trials. 2021. PMID: 34001174 Free PMC article.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review.Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2. PMID: 32406927 Free PMC article. Updated.
-
Vitamin D supplementation for the treatment of COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 May 24;5(5):CD015043. doi: 10.1002/14651858.CD015043. Cochrane Database Syst Rev. 2021. PMID: 34029377 Free PMC article.
Cited by
-
Therapeutic implications of quercetin and its derived-products in COVID-19 protection and prophylactic.Heliyon. 2024 Apr 30;10(9):e30080. doi: 10.1016/j.heliyon.2024.e30080. eCollection 2024 May 15. Heliyon. 2024. PMID: 38765079 Free PMC article. Review.
References
-
- Ader F, Bouscambert-Duchamp M, Hites M, Peiffer-Smadja N, Poissy J, Belhadi D, et al. . Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis. (2022) 22:209–21. doi: 10.1016/S1473-3099(21)00485-0, PMID: - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

