Physicochemical, technofunctional, in vitro antioxidant, and in situ muscle protein synthesis properties of a sprat (Sprattus sprattus) protein hydrolysate
- PMID: 37426190
- PMCID: PMC10328741
- DOI: 10.3389/fnut.2023.1197274
Physicochemical, technofunctional, in vitro antioxidant, and in situ muscle protein synthesis properties of a sprat (Sprattus sprattus) protein hydrolysate
Abstract
Introduction: Sprat (Sprattus sprattus) is an underutilized fish species that may act as an economic and sustainable alternative source of protein due to its good amino acid (AA) profile along with its potential to act as a source of multiple bioactive peptide sequences.
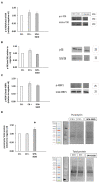
Method and results: This study characterized the physicochemical, technofunctional, and in vitro antioxidant properties along with the AA profile and score of a sprat protein enzymatic hydrolysate (SPH). Furthermore, the impact of the SPH on the growth, proliferation, and muscle protein synthesis (MPS) in skeletal muscle (C2C12) myotubes was examined. The SPH displayed good solubility and emulsion stabilization properties containing all essential and non-essential AAs. Limited additional hydrolysis was observed following in vitro-simulated gastrointestinal digestion (SGID) of the SPH. The SGID-treated SPH (SPH-SGID) displayed in vitro oxygen radical antioxidant capacity (ORAC) activity (549.42 μmol TE/g sample) and the ability to reduce (68%) reactive oxygen species (ROS) production in C2C12 myotubes. Muscle growth and myotube thickness were analyzed using an xCELLigence™ platform in C2C12 myotubes treated with 1 mg protein equivalent.mL-1 of SPH-SGID for 4 h. Anabolic signaling (phosphorylation of mTOR, rpS6, and 4E-BP1) and MPS (measured by puromycin incorporation) were assessed using immunoblotting. SPH-SGID significantly increased myotube thickness (p < 0.0001) compared to the negative control (cells grown in AA and serum-free medium). MPS was also significantly higher after incubation with SPH-SGID compared with the negative control (p < 0.05).
Conclusions: These preliminary in situ results indicate that SPH may have the ability to promote muscle enhancement. In vivo human studies are required to verify these findings.
Keywords: C2C12 cells; antioxidant; muscle growth; muscle protein synthesis; sprat protein hydrolysates; technofunctional.
Copyright © 2023 Shekoohi, Naik, Amigo-Benavent, Harnedy-Rothwell, Carson and FitzGerald.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- FAO . The State of World Fisheries and Aquaculture 2016. Contributing to Food Security and Nutrition for All. Rome: FAO; (2016).
-
- Van Anrooy R, Espinoza Córdova F, Japp D, Valderrama D, Gopal Karmakar K, Lengyel P, et al. . World Review of Capture Fisheries and Aquaculture Insurance 2022. Food & Agriculture Org. (2022).
-
- ŠliŽyte R, Rustad T, Storrø I. Enzymatic hydrolysis of cod (Gadus morhua) by-products: optimization of yield and properties of lipid and protein fractions. Process Biochem. (2005) 40:3680–92. 10.1016/j.procbio.2005.04.007 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

