The toxic metal hypothesis for neurological disorders
- PMID: 37426441
- PMCID: PMC10328356
- DOI: 10.3389/fneur.2023.1173779
The toxic metal hypothesis for neurological disorders
Abstract
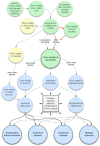
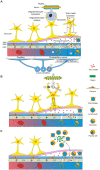
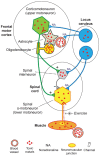
Multiple sclerosis and the major sporadic neurogenerative disorders, amyotrophic lateral sclerosis, Parkinson disease, and Alzheimer disease are considered to have both genetic and environmental components. Advances have been made in finding genetic predispositions to these disorders, but it has been difficult to pin down environmental agents that trigger them. Environmental toxic metals have been implicated in neurological disorders, since human exposure to toxic metals is common from anthropogenic and natural sources, and toxic metals have damaging properties that are suspected to underlie many of these disorders. Questions remain, however, as to how toxic metals enter the nervous system, if one or combinations of metals are sufficient to precipitate disease, and how toxic metal exposure results in different patterns of neuronal and white matter loss. The hypothesis presented here is that damage to selective locus ceruleus neurons from toxic metals causes dysfunction of the blood-brain barrier. This allows circulating toxicants to enter astrocytes, from where they are transferred to, and damage, oligodendrocytes, and neurons. The type of neurological disorder that arises depends on (i) which locus ceruleus neurons are damaged, (ii) genetic variants that give rise to susceptibility to toxic metal uptake, cytotoxicity, or clearance, (iii) the age, frequency, and duration of toxicant exposure, and (iv) the uptake of various mixtures of toxic metals. Evidence supporting this hypothesis is presented, concentrating on studies that have examined the distribution of toxic metals in the human nervous system. Clinicopathological features shared between neurological disorders are listed that can be linked to toxic metals. Details are provided on how the hypothesis applies to multiple sclerosis and the major neurodegenerative disorders. Further avenues to explore the toxic metal hypothesis for neurological disorders are suggested. In conclusion, environmental toxic metals may play a part in several common neurological disorders. While further evidence to support this hypothesis is needed, to protect the nervous system it would be prudent to take steps to reduce environmental toxic metal pollution from industrial, mining, and manufacturing sources, and from the burning of fossil fuels.
Keywords: Alzheimer disease; Parkinson disease; amyotrophic lateral sclerosis; astrocytes; locus ceruleus; multiple sclerosis; oligodendrocytes; toxic metals.
Copyright © 2023 Pamphlett and Bishop.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Mercury is present in neurons and oligodendrocytes in regions of the brain affected by Parkinson's disease and co-localises with Lewy bodies.PLoS One. 2022 Jan 11;17(1):e0262464. doi: 10.1371/journal.pone.0262464. eCollection 2022. PLoS One. 2022. PMID: 35015796 Free PMC article.
-
Concentrations of toxic metals and essential trace elements vary among individual neurons in the human locus ceruleus.PLoS One. 2020 May 19;15(5):e0233300. doi: 10.1371/journal.pone.0233300. eCollection 2020. PLoS One. 2020. PMID: 32428015 Free PMC article.
-
Uptake of environmental toxicants by the locus ceruleus: a potential trigger for neurodegenerative, demyelinating and psychiatric disorders.Med Hypotheses. 2014 Jan;82(1):97-104. doi: 10.1016/j.mehy.2013.11.016. Epub 2013 Nov 21. Med Hypotheses. 2014. PMID: 24315447
-
Implications of metal accumulation mechanisms to phytoremediation.Environ Sci Pollut Res Int. 2009 Mar;16(2):162-75. doi: 10.1007/s11356-008-0079-z. Epub 2008 Dec 6. Environ Sci Pollut Res Int. 2009. PMID: 19067014 Review.
-
Review of Amyotrophic Lateral Sclerosis, Parkinson's and Alzheimer's diseases helps further define pathology of the novel paradigm for Alzheimer's with heavy metals as primary disease cause.Med Hypotheses. 2015 Dec;85(6):779-90. doi: 10.1016/j.mehy.2015.10.009. Epub 2015 Oct 19. Med Hypotheses. 2015. PMID: 26604027 Review.
Cited by
-
Heavy Metal Interactions with Neuroglia and Gut Microbiota: Implications for Huntington's Disease.Cells. 2024 Jul 3;13(13):1144. doi: 10.3390/cells13131144. Cells. 2024. PMID: 38994995 Free PMC article. Review.
-
Heavy metal-induced disruption of the autophagy-lysosomal pathway: implications for aging and neurodegenerative disorders.Biometals. 2025 Apr;38(2):371-417. doi: 10.1007/s10534-025-00665-x. Epub 2025 Feb 17. Biometals. 2025. PMID: 39960543 Review.
-
Early life events may be the first steps on the multistep path to amyotrophic lateral sclerosis.Sci Rep. 2024 Nov 18;14(1):28497. doi: 10.1038/s41598-024-78240-6. Sci Rep. 2024. PMID: 39557859 Free PMC article.
-
Iron Overload, Microbleeding and the Role of Bilirubin in Alzheimer's Disease Brain: Revisiting the Vascular Hypothesis.Int J Mol Sci. 2025 Mar 27;26(7):3060. doi: 10.3390/ijms26073060. Int J Mol Sci. 2025. PMID: 40243777 Free PMC article. Review.
-
Elemental biomapping of human tissues suggests toxic metals such as mercury play a role in the pathogenesis of cancer.Front Oncol. 2024 Jun 21;14:1420451. doi: 10.3389/fonc.2024.1420451. eCollection 2024. Front Oncol. 2024. PMID: 38974240 Free PMC article.
References
LinkOut - more resources
Full Text Sources

