Immuno-PET Imaging of CD69 Visualizes T-Cell Activation and Predicts Survival Following Immunotherapy in Murine Glioblastoma
- PMID: 37426447
- PMCID: PMC10324623
- DOI: 10.1158/2767-9764.CRC-22-0434
Immuno-PET Imaging of CD69 Visualizes T-Cell Activation and Predicts Survival Following Immunotherapy in Murine Glioblastoma
Abstract
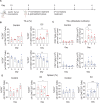
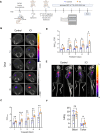
Glioblastoma (GBM) is the most common and malignant primary brain tumor in adults. Immunotherapy may be promising for the treatment of some patients with GBM; however, there is a need for noninvasive neuroimaging techniques to predict immunotherapeutic responses. The effectiveness of most immunotherapeutic strategies requires T-cell activation. Therefore, we aimed to evaluate an early marker of T-cell activation, CD69, for its use as an imaging biomarker of response to immunotherapy for GBM. Herein, we performed CD69 immunostaining on human and mouse T cells following in vitro activation and post immune checkpoint inhibitors (ICI) in an orthotopic syngeneic mouse glioma model. CD69 expression on tumor-infiltrating leukocytes was assessed using single-cell RNA sequencing (scRNA-seq) data from patients with recurrent GBM receiving ICI. Radiolabeled CD69 Ab PET/CT imaging (CD69 immuno-PET) was performed on GBM-bearing mice longitudinally to quantify CD69 and its association with survival following immunotherapy. We show CD69 expression is upregulated upon T-cell activation and on tumor-infiltrating lymphocytes (TIL) in response to immunotherapy. Similarly, scRNA-seq data demonstrated elevated CD69 on TILs from patients with ICI-treated recurrent GBM as compared with TILs from control cohorts. CD69 immuno-PET studies showed a significantly higher tracer uptake in the tumors of ICI-treated mice compared with controls. Importantly, we observed a positive correlation between survival and CD69 immuno-PET signals in immunotherapy-treated animals and established a trajectory of T-cell activation by virtue of CD69-immuno-PET measurements. Our study supports the potential use of CD69 immuno-PET as an immunotherapy response assessment imaging tool for patients with GBM.
Significance: Immunotherapy may hold promise for the treatment of some patients with GBM. There is a need to assess therapy responsiveness to allow the continuation of effective treatment in responders and to avoid ineffective treatment with potential adverse effects in the nonresponders. We demonstrate that noninvasive PET/CT imaging of CD69 may allow early detection of immunotherapy responsiveness in patients with GBM.
© 2023 The Authors; Published by the American Association for Cancer Research.
Figures



References
-
- Delgado-López PD, Corrales-García EM. Survival in glioblastoma: a review on the impact of treatment modalities. Clin Transl Oncol 2016;18:1062–71. - PubMed
-
- Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, et al. . Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A randomized clinical trial. JAMA 2015;314:2535–43. - PubMed
-
- Chen K-T, Wu T-WE, Chuang C-C, Hsu Y-H, Hsu P-W, Huang Y-C, et al. . Corpus callosum involvement and postoperative outcomes of patients with gliomas. J Neurooncol 2015;124:207–14. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

