Organic Controls over Biomineral Ca-Mg Carbonate Compositions and Morphologies
- PMID: 37426546
- PMCID: PMC10326858
- DOI: 10.1021/acs.cgd.3c00102
Organic Controls over Biomineral Ca-Mg Carbonate Compositions and Morphologies
Abstract
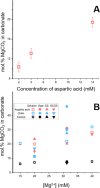
Calcium carbonate minerals, such as aragonite and calcite, are widespread in biomineral skeletons, shells, exoskeletons, and more. With rapidly increasing pCO2 levels linked to anthropogenic climate change, carbonate minerals face the threat of dissolution, especially in an acidifying ocean. Given the right conditions, Ca-Mg carbonates (especially disordered dolomite and dolomite) are alternative minerals for organisms to utilize, with the added benefit of being harder and more resistant to dissolution. Ca-Mg carbonate also holds greater potential for carbon sequestration due to both Ca and Mg cations being available to bond with the carbonate group (CO32-). However, Mg-bearing carbonates are relatively rare biominerals because the high kinetic energy barrier for the dehydration of the Mg2+-water complex severely restricts Mg incorporation in carbonates at Earth surface conditions. This work presents the first overview of the effects of the physiochemical properties of amino acids and chitins on the mineralogy, composition, and morphology of Ca-Mg carbonates in solutions and on solid surfaces. We discovered that acidic, negatively charged, hydrophilic amino acids (aspartic and glutamic) and chitins could induce the precipitation of high-magnesium calcite (HMC) and disordered dolomite in solution and on solid surfaces with these adsorbed biosubstrates via in vitro experiments. Thus, we expect that acidic amino acids and chitins are among the controlling factors in biomineralization used in different combinations to control the mineral phases, compositions, and morphologies of Ca-Mg carbonate biomineral crystals.
© 2023 The Authors and Smithsonian Institution. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Knoll A. H. Biomineralization and Evolutionary History. Rev. Mineral. Geochem. 2003, 54, 329–356. 10.2113/0540329. - DOI
-
- Langer G.; Geisen M.; Baumann K. H.; Kläs J.; Riebesell U.; Thoms S.; Young J. R. Species-Specific Responses of Calcifying Algae to Changing Seawater Carbonate Chemistry. Geochem., Geophys. Geosystems 2006, 7, 01227 10.1029/2005GC001227. - DOI
-
- Schiebel R. Planktic Foraminiferal Sedimentation and the Marine Calcite Budget. Global Biogeochem. Cycles 2002, 16, 3-1–3-21. 10.1029/2001gb001459. - DOI
LinkOut - more resources
Full Text Sources
