Combination of thalidomide and Clostridium butyricum relieves chemotherapy-induced nausea and vomiting via gut microbiota and vagus nerve activity modulation
- PMID: 37426650
- PMCID: PMC10327820
- DOI: 10.3389/fimmu.2023.1220165
Combination of thalidomide and Clostridium butyricum relieves chemotherapy-induced nausea and vomiting via gut microbiota and vagus nerve activity modulation
Abstract
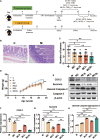
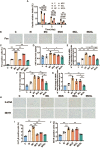
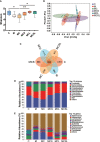
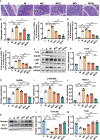
Nausea and vomiting (CINV) are distressful and widespread side effects of chemotherapy, and additional efficient regimens to alleviate CINV are urgently needed. In the present study, colorectal cancer (CRC) mice model induced by Azoxymethane (AOM)/Dextran Sodium Sulfate (DSS) was employed to evaluate the cancer suppression and CINV amelioration effect of the combination of thalidomide (THD) and Clostridium butyricum. Our results suggested that the combination of THD and C. butyricum abundantly enhanced the anticancer effect of cisplatin via activating the caspase-3 apoptosis pathway, and also ameliorated CINV via inhibiting the neurotransmitter (e.g., 5-HT and tachykinin 1) and its receptor (e.g., 5-HT3R and NK-1R) in brain and colon. Additionally, the combination of THD and C. butyricum reversed the gut dysbacteriosis in CRC mice by increasing the abundance of Clostridium, Lactobacillus, Bifidobacterium, and Ruminococcus at the genus level, and also led to increased expression of occludin and Trek1 in the colon, while decreased expression of TLR4, MyD88, NF-κB, and HDAC1, as well as the mRNA level of IL-6, IL-1β, and TNF-α. In all, these results suggest that the combination of THD and C. butyricum had good efficacy in enhancing cancer treatments and ameliorating CINV, which thus provides a more effective strategy for the treatment of CRC.
Keywords: CINV; Clostridium butyricum; gut- brain axis; intestinal microecology; thalidomide.
Copyright © 2023 Zhao, Wu, Zhu, Shang, Wei, Shang, Tian, Chen and Wei.
Conflict of interest statement
Author GS was employed by Eastsea Pharma Co. LTD. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Clostridium butyricum modulates gut microbiota and reduces colitis associated colon cancer in mice.Int Immunopharmacol. 2020 Nov;88:106862. doi: 10.1016/j.intimp.2020.106862. Epub 2020 Aug 6. Int Immunopharmacol. 2020. PMID: 32771947
-
Clostridium butyricum protects the epithelial barrier by maintaining tight junction protein expression and regulating microflora in a murine model of dextran sodium sulfate-induced colitis.Scand J Gastroenterol. 2018 Sep;53(9):1031-1042. doi: 10.1080/00365521.2016.1192678. Epub 2018 Aug 24. Scand J Gastroenterol. 2018. PMID: 30141701
-
Clostridium butyricum and Chitooligosaccharides in Synbiotic Combination Ameliorate Symptoms in a DSS-Induced Ulcerative Colitis Mouse Model by Modulating Gut Microbiota and Enhancing Intestinal Barrier Function.Microbiol Spectr. 2023 Mar 28;11(2):e0437022. doi: 10.1128/spectrum.04370-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36975838 Free PMC article.
-
Efficacy and Safety of Thalidomide As a Pre-Medication of Chemotherapy-Induced Nausea and Vomiting (CINV) Following Highly Emetogenic Chemotherapy (HEC): A Systematic Review and Meta-Analysis.Front Oncol. 2022 Jan 24;11:818839. doi: 10.3389/fonc.2021.818839. eCollection 2021. Front Oncol. 2022. PMID: 35141156 Free PMC article.
-
The relationship between Clostridium butyricum and colorectal cancer.J Cancer Res Ther. 2022 Dec;18(7):1855-1859. doi: 10.4103/jcrt.jcrt_1565_21. J Cancer Res Ther. 2022. PMID: 36647942 Review.
Cited by
-
Clostridium butyricum regulates intestinal barrier function via trek1 to improve behavioral abnormalities in mice with autism spectrum disorder.Cell Biosci. 2024 Jul 21;14(1):95. doi: 10.1186/s13578-024-01278-6. Cell Biosci. 2024. PMID: 39034406 Free PMC article.
-
Tocotrienol suppresses colitis-associated cancer progression through TLR4 signaling in a mouse model of colorectal cancer.Curr Res Toxicol. 2024 Sep 26;7:100196. doi: 10.1016/j.crtox.2024.100196. eCollection 2024. Curr Res Toxicol. 2024. PMID: 39411685 Free PMC article.
-
Operationalizing Team Science at the Academic Cancer Center Network to Unveil the Structure and Function of the Gut Microbiome.J Clin Med. 2025 Mar 17;14(6):2040. doi: 10.3390/jcm14062040. J Clin Med. 2025. PMID: 40142848 Free PMC article. Review.
-
Comprehensive gut microbiota and metabolomics combined with network pharmacology reveal the effects of acupuncture treatment for chemotherapy-induced nausea and vomiting.Transl Gastroenterol Hepatol. 2025 Apr 14;10:26. doi: 10.21037/tgh-24-35. eCollection 2025. Transl Gastroenterol Hepatol. 2025. PMID: 40337760 Free PMC article.
-
The Gut Microbial Lipid Metabolite 14(15)-EpETE Inhibits Substance P Release by Targeting GCG/PKA Signaling to Relieve Cisplatin-Induced Nausea and Vomiting in Rats.J Microbiol Biotechnol. 2024 Sep 28;34(9):1769-1777. doi: 10.4014/jmb.2403.03044. Epub 2024 Jul 15. J Microbiol Biotechnol. 2024. PMID: 39187454 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

