Three-dimensional genome structure and function
- PMID: 37426677
- PMCID: PMC10329473
- DOI: 10.1002/mco2.326
Three-dimensional genome structure and function
Abstract
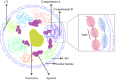
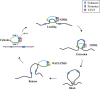
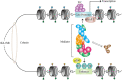
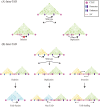
Linear DNA undergoes a series of compression and folding events, forming various three-dimensional (3D) structural units in mammalian cells, including chromosomal territory, compartment, topologically associating domain, and chromatin loop. These structures play crucial roles in regulating gene expression, cell differentiation, and disease progression. Deciphering the principles underlying 3D genome folding and the molecular mechanisms governing cell fate determination remains a challenge. With advancements in high-throughput sequencing and imaging techniques, the hierarchical organization and functional roles of higher-order chromatin structures have been gradually illuminated. This review systematically discussed the structural hierarchy of the 3D genome, the effects and mechanisms of cis-regulatory elements interaction in the 3D genome for regulating spatiotemporally specific gene expression, the roles and mechanisms of dynamic changes in 3D chromatin conformation during embryonic development, and the pathological mechanisms of diseases such as congenital developmental abnormalities and cancer, which are attributed to alterations in 3D genome organization and aberrations in key structural proteins. Finally, prospects were made for the research about 3D genome structure, function, and genetic intervention, and the roles in disease development, prevention, and treatment, which may offer some clues for precise diagnosis and treatment of related diseases.
Keywords: cancer; congenital developmental abnormality; three‐dimensional genome; topologically associating domain.
© 2023 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Understanding 3D Genome Organization and Its Effect on Transcriptional Gene Regulation Under Environmental Stress in Plant: A Chromatin Perspective.Front Cell Dev Biol. 2021 Dec 8;9:774719. doi: 10.3389/fcell.2021.774719. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34957106 Free PMC article. Review.
-
Molecular mechanism of the 3D genome structure and function regulation during cell terminal differentiation.Yi Chuan. 2020 Jan 20;42(1):32-44. doi: 10.16288/j.yczz.19-270. Yi Chuan. 2020. PMID: 31956095 Review.
-
Three-dimensional genome landscape comprehensively reveals patterns of spatial gene regulation in papillary and anaplastic thyroid cancers: a study using representative cell lines for each cancer type.Cell Mol Biol Lett. 2023 Jan 6;28(1):1. doi: 10.1186/s11658-022-00409-6. Cell Mol Biol Lett. 2023. PMID: 36609218 Free PMC article.
-
The role of 3D genome organization in development and cell differentiation.Nat Rev Mol Cell Biol. 2019 Sep;20(9):535-550. doi: 10.1038/s41580-019-0132-4. Nat Rev Mol Cell Biol. 2019. PMID: 31197269 Review.
-
Deciphering the 3D structures of plant genomes: Building blocks of hierarchically organized chromatin domains.J Exp Bot. 2025 May 20:eraf218. doi: 10.1093/jxb/eraf218. Online ahead of print. J Exp Bot. 2025. PMID: 40391535
Cited by
-
Inherited Genetic Variation in Parkinson's Disease: Convergence on Impaired Autophagosome-Lysosome Fusion Through the Altered Expression of mRNA Isoforms.Mol Neurobiol. 2025 Jun 2. doi: 10.1007/s12035-025-05101-2. Online ahead of print. Mol Neurobiol. 2025. PMID: 40457026
-
Allele-Specific Regulation of the Candidate Autism Liability Gene RAI1 by the Enhancer Variant rs4925102 (C/G).Genes (Basel). 2024 Apr 6;15(4):460. doi: 10.3390/genes15040460. Genes (Basel). 2024. PMID: 38674394 Free PMC article.
-
Node features of chromosome structure networks and their connections to genome annotation.Comput Struct Biotechnol J. 2024 May 17;23:2240-2250. doi: 10.1016/j.csbj.2024.05.026. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 38827231 Free PMC article.
-
Unveiling epigenetic regulatory elements associated with breast cancer development.bioRxiv [Preprint]. 2024 Nov 15:2024.11.12.623187. doi: 10.1101/2024.11.12.623187. bioRxiv. 2024. Update in: Int J Mol Sci. 2025 Jul 08;26(14):6558. doi: 10.3390/ijms26146558. PMID: 39605637 Free PMC article. Updated. Preprint.
-
Epigenetic regulation of cardiovascular diseases induced by behavioral and environmental risk factors: Mechanistic, diagnostic, and therapeutic insights.FASEB Bioadv. 2024 Oct 1;6(11):477-502. doi: 10.1096/fba.2024-00080. eCollection 2024 Nov. FASEB Bioadv. 2024. PMID: 39512842 Free PMC article. Review.
References
-
- Stadhouders R, Filion GJ, Graf T. Transcription factors and 3D genome conformation in cell‐fate decisions. Nature. 2019;569(7756):345‐354. - PubMed
-
- Cremer T, Cremer C. Rise, fall and resurrection of chromosome territories: a historical perspective. Part I. The rise of chromosome territories. European Journal of Histochemistry: EJH. 2006;50(3):161‐176. - PubMed
-
- Cremer T, Cremer C. Rise, fall and resurrection of chromosome territories: a historical perspective. Part II. Fall and resurrection of chromosome territories during the 1950s to 1980s. Part III. Chromosome territories and the functional nuclear architecture: experiments and models from the 1990s to the present. European Journal of Histochemistry: EJH. 2006;50(4):223‐272. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
