Unraveling the reaction mechanisms for furfural electroreduction on copper
- PMID: 37426696
- PMCID: PMC10323714
- DOI: 10.1039/d3ey00040k
Unraveling the reaction mechanisms for furfural electroreduction on copper
Abstract
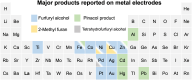
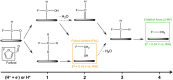
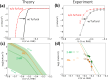
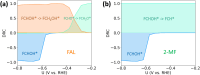
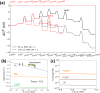
Electrochemical routes for the valorization of biomass-derived feedstock molecules offer sustainable pathways to produce chemicals and fuels. However, the underlying reaction mechanisms for their electrochemical conversion remain elusive. In particular, the exact role of proton-electron coupled transfer and electrocatalytic hydrogenation in the reaction mechanisms for biomass electroreduction are disputed. In this work, we study the reaction mechanism underlying the electroreduction of furfural, an important biomass-derived platform chemical, combining grand-canonical (constant-potential) density functional theory-based microkinetic simulations and pH dependent experiments on Cu under acidic conditions. Our simulations indicate the second PCET step in the reaction pathway to be the rate- and selectivity-determining step for the production of the two main products of furfural electroreduction on Cu, i.e., furfuryl alcohol and 2-methyl furan, at moderate overpotentials. We further identify the source of Cu's ability to produce both products with comparable activity in their nearly equal activation energies. Furthermore, our microkinetic simulations suggest that surface hydrogenation steps play a minor role in determining the overall activity of furfural electroreduction compared to PCET steps due to the low steady-state hydrogen coverage predicted under reaction conditions, the high activation barriers for surface hydrogenation and the observed pH dependence of the reaction. As a theoretical guideline, low pH (<1.5) and moderate potential (ca. -0.5 V vs. SHE) conditions are suggested for selective 2-MF production.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


References
-
- Harnisch F. Urban C. Electrobiorefineries: Unlocking the Synergy of Electrochemical and Microbial Conversions. Angew. Chem., Int. Ed. 2018;57(32):10016–10023. doi: 10.1002/anie.201711727. https://dx.doi.org/10.1002/anie.201711727 - DOI - DOI - PubMed
-
- Akhade S. A. Singh N. Gutiérrez O. Y. Lopez-Ruiz J. Wang H. Holladay J. D. Liu Y. Karkamkar A. Weber R. S. Padmaperuma A. B. Lee M.-S. Whyatt G. A. Elliott M. Holladay J. E. Male J. L. Lercher J. A. Rousseau R. Glezakou V.-A. Electrocatalytic Hydrogenation of Biomass-Derived Organics: A Review. Chem. Rev. 2020;120(20):11370–11419. doi: 10.1021/acs.chemrev.0c00158. https://dx.doi.org/10.1021/acs.chemrev.0c00158 - DOI - DOI - PubMed
-
- Mariscal R. Maireles-Torres P. Ojeda M. Sádaba I. López Granados M. Furfural: A Renewable and Versatile Platform Molecule for the Synthesis of Chemicals and Fuels. Energy Environ. Sci. 2016;9(4):1144–1189. doi: 10.1039/C5EE02666K. https://dx.doi.org/10.1039/C5EE02666K - DOI - DOI
-
- Liu S. Govindarajan N. Chan K. Understanding Activity Trends in Furfural Hydrogenation on Transition Metal Surfaces. ACS Catal. 2022:12902–12910. doi: 10.1021/acscatal.2c03822. https://dx.doi.org/10.1021/acscatal.2c03822 - DOI - DOI
-
- Albert W. C. Lowy A. The Electrochemical Reduction of Furfural. Trans. Electrochem. Soc. 1939;75(1):367. doi: 10.1149/1.3498392. https://dx.doi.org/10.1149/1.3498392 - DOI - DOI
LinkOut - more resources
Full Text Sources
Research Materials
