Genotyping of Patients with Adverse Drug Reaction or Therapy Failure: Database Analysis of a Pharmacogenetics Case Series Study
- PMID: 37426898
- PMCID: PMC10327911
- DOI: 10.2147/PGPM.S415259
Genotyping of Patients with Adverse Drug Reaction or Therapy Failure: Database Analysis of a Pharmacogenetics Case Series Study
Abstract
Purpose: Pharmacogenetics (PGx) is an emerging aspect of personalized medicine with the potential to increase efficacy and safety of pharmacotherapy. However, PGx testing is still not routinely integrated into clinical practice. We conducted an observational case series study where PGx information from a commercially available panel test covering 30 genes was integrated into medication reviews. The aim of the study was to identify the drugs that are most frequently object of drug-gene-interactions (DGI) in the study population.
Patients and methods: In out-patient and in-patient settings, we recruited 142 patients experiencing adverse drug reaction (ADR) and/or therapy failure (TF). Collected anonymized data from the individual patient was harmonized and transferred to a structured database.
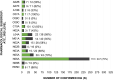
Results: The majority of the patients had a main diagnosis of a mental or behavioral disorder (ICD-10: F, 61%), of musculoskeletal system and connective tissue diseases (ICD-10: M, 21%), and of the circulatory system (ICD-10: I, 11%). The number of prescribed medicines reached a median of 7 per person, resulting in a majority of patients with polypharmacy (≥5 prescribed medicines, 65%). In total, 559 suspected DGI were identified in 142 patients. After genetic testing, an association with at least one genetic variation was confirmed for 324 suspected DGI (58%) caused by 64 different drugs and 21 different genes in 141 patients. After 6 months, PGx-based medication adjustments were recorded for 62% of the study population, whereby differences were identified in subgroups.
Conclusion: The data analysis from this study provides valuable insights for the main focus of further research in the context of PGx. The results indicate that most of the selected patients in our sample represent suitable target groups for PGx panel testing in clinical practice, notably those taking drugs for mental or behavioral disorder, circulatory diseases, immunological diseases, pain-related diseases, and patients experiencing polypharmacy.
Keywords: PGx; clinical pharmacy; clinical practice; medication review; personalized medicine; pharmacogenomics.
© 2023 Bollinger et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures

Similar articles
-
Polypharmacy and pharmacogenomics in high-acuity behavioral health care for autism spectrum disorder: a retrospective study.Child Adolesc Psychiatry Ment Health. 2025 May 21;19(1):60. doi: 10.1186/s13034-025-00915-3. Child Adolesc Psychiatry Ment Health. 2025. PMID: 40399951 Free PMC article.
-
Identifying the prevalence of clinically actionable drug-gene interactions in a health system biorepository to guide pharmacogenetics implementation services.Clin Transl Sci. 2023 Feb;16(2):292-304. doi: 10.1111/cts.13449. Epub 2022 Dec 12. Clin Transl Sci. 2023. PMID: 36510710 Free PMC article.
-
Clinical implementation of preemptive pharmacogenomics in psychiatry.EBioMedicine. 2024 Mar;101:105009. doi: 10.1016/j.ebiom.2024.105009. Epub 2024 Feb 16. EBioMedicine. 2024. PMID: 38364700 Free PMC article.
-
Implementation of Pharmacogenomics in Everyday Clinical Settings.Adv Pharmacol. 2018;83:219-246. doi: 10.1016/bs.apha.2018.04.003. Adv Pharmacol. 2018. PMID: 29801576 Review.
-
The medical and economic roles of pipeline pharmacogenetics: Alzheimer's disease as a model of efficacy and HLA-B(*)5701 as a model of safety.Neuropsychopharmacology. 2009 Jan;34(1):6-17. doi: 10.1038/npp.2008.153. Epub 2008 Oct 15. Neuropsychopharmacology. 2009. PMID: 18923406 Review.
Cited by
-
Impact and Enablers of Pharmacogenetic-Informed Treatment Decisions-A Longitudinal Mixed-Methods Study Exploring the Patient Perspective.Pharmacy (Basel). 2025 Jan 31;13(1):14. doi: 10.3390/pharmacy13010014. Pharmacy (Basel). 2025. PMID: 39998012 Free PMC article.
-
Association of Pharmacogenotyping and Patient-Reported Outcomes in Chronic Pain Management.Health Serv Insights. 2025 Jul 12;18:11786329251356560. doi: 10.1177/11786329251356560. eCollection 2025. Health Serv Insights. 2025. PMID: 40655117 Free PMC article.
-
Overcoming Barriers: Strategies for Implementing Pharmacist-Led Pharmacogenetic Services in Swiss Clinical Practice.Genes (Basel). 2024 Jul 1;15(7):862. doi: 10.3390/genes15070862. Genes (Basel). 2024. PMID: 39062642 Free PMC article.
-
Real-World Utilization of Medications With Pharmacogenetic Recommendations in Older Adults: A Scoping Review.Clin Transl Sci. 2025 Feb;18(2):e70126. doi: 10.1111/cts.70126. Clin Transl Sci. 2025. PMID: 39967300 Free PMC article.
References
-
- National Institute for Health and Care Excellence (NICE). Nice guideline. medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes; 2015. Available from: https://www.nice.org.uk/guidance/ng5. Accessed June 5, 2023. - PubMed
-
- World Health Organization (WHO). Medication safety in polypharmacy; 2019. Available from: https://www.who.int/publications/i/item/WHO-UHC-SDS-2019.11. Accessed June 5, 2023.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

