Radiation and Dose-densification of R-CHOP in Aggressive B-cell Lymphoma With Intermediate Prognosis: The UNFOLDER Study
- PMID: 37427146
- PMCID: PMC10325769
- DOI: 10.1097/HS9.0000000000000904
Radiation and Dose-densification of R-CHOP in Aggressive B-cell Lymphoma With Intermediate Prognosis: The UNFOLDER Study
Abstract
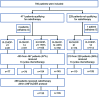
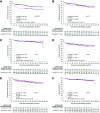
UNFOLDER (Unfavorable Young Low-Risk Densification of R-Chemo Regimens) is an international phase-3 trial in patients 18-60 years with aggressive B-cell lymphoma and intermediate prognosis defined by age-adjusted International Prognostic Index (aaIPI) of 0 and bulky disease (≥7.5 cm) or aaIPI of 1. In a 2 × 2 factorial design patients were randomized to 6× R-CHOP-14 or 6× R-CHOP-21 (rituximab, cyclophosphamide, doxorubicin, vincristine, and prediso[lo]ne) and to consolidation radiotherapy to extralymphatic and bulky disease or observation. Response was assessed according to the standardized response criteria published in 1999, not including F-18 fluordesoxyglucose positron emission tomography/computed tomography (FDG-PET). Primary endpoint was event-free survival (EFS). A total of 695 of 700 patients were eligible for the intention-to-treat analysis. Totally 467 patients qualified for radiotherapy of whom 305 patients were randomized to receive radiotherapy (R-CHOP-21: 155; R-CHOP-14: 150) and 162 to observation (R-CHOP-21: 81, R-CHOP-14: 81). Two hundred twenty-eight patients not qualifying for radiotherapy were randomized for R-CHOP-14 versus R-CHOP-21. After a median observation of 66 months 3-year EFS was superior in the radiotherapy-arm versus observation-arm (84% versus 68%; P = 0.0012), due to a lower rate of partial responses (PR) (2% versus 11%). PR often triggered additional treatment, mostly radiotherapy. No significant difference was observed in progression-free survival (PFS) (89% versus 81%; P = 0.22) and overall survival (OS) (93% versus 93%; P = 0.51). Comparing R-CHOP-14 and R-CHOP-21 EFS, PFS and OS were not different. Patients randomized to radiotherapy had a superior EFS, largely due to a lower PR rate requiring less additional treatment (NCT00278408, EUDRACT 2005-005218-19).
Copyright © 2023 the Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the European Hematology Association.
Conflict of interest statement
L. Thurner has received travel grants from Abbvie, Janssen and EUSA-Pharm, and has indicated consultancy for Takeda, Astra-Zeneca, Merck, EUSA-pharm. DK-M has received personal fees from Novartis, Astra-Zeneca, Gilead Sciences, GlaxoSmithKline, Janssen-Cilag, and nonfinancial support from Gilead Sciences, Janssen-Cilag, Takeda, Novartis. AV has received honoraria from Roche, Amgen, Kite, Gilead, Novartis, Bristol-Myers Squibb and has indicated a membership of the advisory board of Roche, Amgen, Kite, Gilead, Novartis, Bristol-Myers Squibb. UBK has received honoraria and advisory fees from Roche, Janssen-Cilag, Takeda, Bristol-Myers Squibb, Gilead, Hexal, Pfizer, Astra- Zeneca, Pentixapharm, Amgen, Novartis, MSD and has received clinical study support for Celgene, Takeda, BMS, Roche, Astra-Zeneca, Novartis, MSD, Janssen-Cilag, Pfizer. MN has received travel grants from Roche, Celgene and MSD and personal fees from Roche, Celgene, MSD, Janssen, Amgen, Incyte and Abbvie. FG participates in advisory board of Roche, Boehringer Ingelheim, Abbvie, Merck, Takeda, MSD, Sanofi, Pfizer, Novartis, Amgen and Janssen. RT has received grants from Atara and Roche and travel support from Roche, Atara, Celgene, Janssen and Abbvie; he has indicated a membership of the advisory board of Atara and Abbvie and has indicated consultancy for s Atara. PdNB has indicated consultancy for Roche, Incyte and Novartis. FM has received travel grants from Roche, Takeda, Janssen and Gilead and has indicated consultancy and advisory role for Roche, Gilead, Janssen, MSD, Takeda and Novartis. SS has received grants from Abbvie, Astra-Zeneca, Celgene, Gilead, Roche, Janssen, Novartis, Morphosys and has indicated consultancy for for Abbvie, Astra-Zeneca, Celgene, Gilead, Roche, Janssen, Novartis, Morphosys; he has received drug/equipment supplied by entity from Abbvie, Astra-Zeneca, Celgene, Gilead, Roche, Janssen, Novartis, Morphosys. VP has received grants from Deutsche Krebshilfe (German Cancer Aid), Chugai, Abbvie, Amgen, Roche and Bristol Myers Squibb. GH has received grants from Roche and Bristol Myers Squibb and personal fees from Bristol Myers Squibb, Roche, Amgen, Spectrum and MSD. All the other authors have no conflicts of interest to disclose.
Figures


References
-
- Coiffier B, Lepage E, Brière J, et al. . CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. - PubMed
-
- Pfreundschuh M, Schubert J, Ziepert M, et al. . Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9:105–116. - PubMed
-
- Pfreundschuh M, Trümper L, Österborg A, et al. . CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. - PubMed
-
- International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–994. - PubMed
-
- Pfreundschuh M, Ho AD, Cavallin-Stahl E, et al. . Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: an exploratory analysis of the MabThera International Trial Group (MInT) study. Lancet Oncol. 2008;9:435–444. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
