Are there reliable multiparametric MRI criteria for differential diagnosis between intracranial meningiomas and solitary intracranial dural metastases?
- PMID: 37427340
- PMCID: PMC10326821
- DOI: 10.3892/ol.2023.13936
Are there reliable multiparametric MRI criteria for differential diagnosis between intracranial meningiomas and solitary intracranial dural metastases?
Abstract
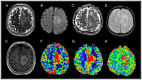
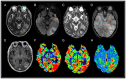
Intracranial meningiomas are the most common tumors of the central nervous system (CNS). Meningiomas account for up to 36% of all brain tumors. The incidence of metastatic brain lesions has not been determined. Up to 30% of adult patients with cancer of one localization or another suffer from a secondary tumor lesion of the brain. The vast majority of meningiomas have meningeal localization; >90% are solitary. The incidence of intracranial dural metastases (IDM) is 8-9% of cases, while in 10% of cases, the brain is the only localization, and in 50% of cases the metastases are solitary. Typically, the task of distinguishing between meningioma and dural metastasis does not involve difficulties. Periodically, there is a situation when the differential diagnosis between these tumors is ambiguous, since meningiomas and solitary IDM may have similar characteristics, in particular, a cavity-less solid structure, limited diffusion of water molecules, the presence of extensive peritumoral edema, and an identical contrast pattern. The present study included 100 patients with newly diagnosed tumors of the CNS, who subsequently underwent examination and neurosurgical treatment at the Federal Center for Neurosurgery with histological verification between May 2019 and October 2022. Depending on the histological conclusion, two study groups of patients were distinguished: The first group consisted of patients diagnosed with intracranial meningiomas (n=50) and the second group of patients were diagnosed with IDM (n=50). The study was performed using a magnetic resonance imaging (MRI) General Electric Discovery W750 3T before and after contrast enhancement. The diagnostic value of this study was estimated using Receiver Operating Characteristic curve and area under the curve analysis. Based on the results of the study, it was found that the use of multiparametric MRI (mpMRI) in the differential diagnosis of intracranial meningiomas and IDM was limited by the similarity of the values of the measured diffusion coefficient. The assumption, previously put forward in the literature, regarding the presence of a statistically significant difference in the apparent diffusion coefficient values, which make it possible to differentiate tumors, was not confirmed. When analyzing perfusion data, IDM showed higher cerebral blood flow (CBF) values compared with intracranial meningiomas (P≤0.001). A threshold value of the CBF index was revealed, which was 217.9 ml/100 g/min, above which it is possible to predict IDM with a sensitivity and specificity of 80.0 and 86.0%, respectively. Diffusion-weighted images are not reliable criteria for differentiating intracranial meningiomas from IDM and should not influence the diagnosis suggested by imaging. The technique for assessing the perfusion of a meningeal lesion makes it possible to predict metastases with a sensitivity and specificity close to 80-90% and deserves attention when making a diagnosis. In the future, in order to reduce the number of false negative and false positive results, mpMRI would require additional criteria to be included in the protocol. Since IDM differs from intracranial meningiomas in the severity of neoangiogenesis and, accordingly, in greater vascular permeability, the technique for assessing vascular permeability (wash-in parameter with dynamic contrast enhancement) may serve as a refining criterion for distinguishing between dural lesions.
Keywords: MR perfusion; biomarkers; diffusion coefficient measurement; intracranial dural metastasis; intracranial meningioma; mpMRI; neuroimaging.
Copyright © 2023, Spandidos Publications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures









Similar articles
-
Quantitative apparent diffusion coefficients in the characterization of brain tumors and associated peritumoral edema.Acta Radiol. 2009 Jul;50(6):682-9. doi: 10.1080/02841850902933123. Acta Radiol. 2009. PMID: 19449234
-
Usefulness of a Rim-Enhancing Pattern on the Contrast-Enhanced 3D-FLAIR Sequence and MRI Characteristics for Distinguishing Meningioma and Malignant Dural-Based Tumor.AJNR Am J Neuroradiol. 2023 Mar;44(3):247-253. doi: 10.3174/ajnr.A7780. Epub 2023 Feb 2. AJNR Am J Neuroradiol. 2023. PMID: 36732030 Free PMC article.
-
Dynamic perfusion MRI characteristics of dural metastases and meningiomas: a pilot study characterizing the first-pass wash-in phase beyond relative cerebral blood volume.AJR Am J Roentgenol. 2011 Apr;196(4):886-90. doi: 10.2214/AJR.10.5309. AJR Am J Roentgenol. 2011. PMID: 21427341
-
Intracranial meningiomas, the VEGF-A pathway, and peritumoral brain oedema.Dan Med J. 2013 Apr;60(4):B4626. Dan Med J. 2013. PMID: 23651727 Review.
-
Contribution of perfusion-weighted magnetic resonance imaging in the differentiation of meningiomas and other extra-axial tumors: case reports and literature review.J Neurooncol. 2011 Jul;103(3):777-83. doi: 10.1007/s11060-010-0445-9. J Neurooncol. 2011. PMID: 21061142 Free PMC article. Review.
Cited by
-
A proposed imaging scoring system to differentiate dural-based metastasis from meningioma using MR and CT images.BMC Med Imaging. 2025 May 15;25(1):163. doi: 10.1186/s12880-025-01723-z. BMC Med Imaging. 2025. PMID: 40375213 Free PMC article.
-
Tumor-to-tumor metastasis of clear cell renal cell carcinoma to meningioma: illustrative case.J Neurosurg Case Lessons. 2025 Mar 10;9(10):CASE24670. doi: 10.3171/CASE24670. Print 2025 Mar 10. J Neurosurg Case Lessons. 2025. PMID: 40064003 Free PMC article.
-
The determination of the boundaries and prediction the radicality of glioblastoma resection using MRI and CT perfusion.Front Neurol. 2025 May 14;16:1572845. doi: 10.3389/fneur.2025.1572845. eCollection 2025. Front Neurol. 2025. PMID: 40438576 Free PMC article.
References
LinkOut - more resources
Full Text Sources
