ADCC-activating antibodies correlate with decreased risk of congenital human cytomegalovirus transmission
- PMID: 37427588
- PMCID: PMC10371338
- DOI: 10.1172/jci.insight.167768
ADCC-activating antibodies correlate with decreased risk of congenital human cytomegalovirus transmission
Abstract
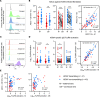
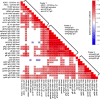
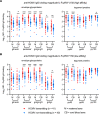
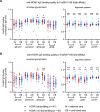
Human cytomegalovirus (HCMV) is the most common vertically transmitted infection worldwide, yet there are no vaccines or therapeutics to prevent congenital HCMV (cCMV) infection. Emerging evidence indicates that antibody Fc effector functions may be a previously underappreciated component of maternal immunity against HCMV. We recently reported that antibody-dependent cellular phagocytosis (ADCP) and IgG activation of FcγRI/FcγRII were associated with protection against cCMV transmission, leading us to hypothesize that additional Fc-mediated antibody functions may be important. In this same cohort of HCMV-transmitting (n = 41) and nontransmitting (n = 40) mother-infant dyads, we report that higher maternal sera antibody-dependent cellular cytotoxicity (ADCC) activation is also associated with lower risk of cCMV transmission. We investigated the relationship between ADCC and IgG responses against 9 viral antigens and found that ADCC activation correlated most strongly with sera IgG binding to the HCMV immunoevasin protein UL16. Moreover, we determined that higher UL16-specific IgG binding and FcγRIII/CD16 engagement were associated with the greatest risk reduction in cCMV transmission. Our findings indicate that ADCC-activating antibodies against targets such as UL16 may represent an important protective maternal immune response against cCMV infection that can guide future HCMV correlates studies and vaccine or antibody-based therapeutic development.
Keywords: Adaptive immunity; Immunoglobulins; Immunology; Infectious disease; NK cells.
Conflict of interest statement
Figures
Update of
-
ADCC-activating antibodies correlate with protection against congenital human cytomegalovirus infection.medRxiv [Preprint]. 2023 Mar 17:2023.03.15.23287332. doi: 10.1101/2023.03.15.23287332. medRxiv. 2023. Update in: JCI Insight. 2023 Jul 10;8(13):e167768. doi: 10.1172/jci.insight.167768. PMID: 36993668 Free PMC article. Updated. Preprint.
References
-
- Gerna G, Lilleri D. Human cytomegalovirus (HCMV) infection/re-infection: development of a protective HCMV vaccine. New Microbiol. 2019;42(1):1–20. - PubMed

