A nondepleting anti-CD19 antibody impairs B cell function and inhibits autoimmune diseases
- PMID: 37427592
- PMCID: PMC10371335
- DOI: 10.1172/jci.insight.166137
A nondepleting anti-CD19 antibody impairs B cell function and inhibits autoimmune diseases
Abstract
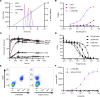
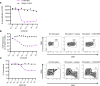
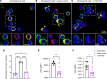
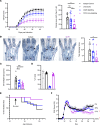
B cells contribute to multiple aspects of autoimmune disorders, and B cell-targeting therapies, including B cell depletion, have been proven to be efficacious in treatment of multiple autoimmune diseases. However, the development of novel therapies targeting B cells with higher efficacy and a nondepleting mechanism of action is highly desirable. Here we describe a nondepleting, high-affinity anti-human CD19 antibody LY3541860 that exhibits potent B cell inhibitory activities. LY3541860 inhibits B cell activation, proliferation, and differentiation of primary human B cells with high potency. LY3541860 also inhibits human B cell activities in vivo in humanized mice. Similarly, our potent anti-mCD19 antibody also demonstrates improved efficacy over CD20 B cell depletion therapy in multiple B cell-dependent autoimmune disease models. Our data indicate that anti-CD19 antibody is a highly potent B cell inhibitor that may have potential to demonstrate improved efficacy over currently available B cell-targeting therapies in treatment of autoimmune conditions without causing B cell depletion.
Keywords: Adaptive immunity; Autoimmunity; Immunoglobulins; Immunology.
Conflict of interest statement
Figures



Comment in
-
Non-depleting anti-CD19 B cell inhibition.Nat Rev Rheumatol. 2023 Sep;19(9):539. doi: 10.1038/s41584-023-01018-z. Nat Rev Rheumatol. 2023. PMID: 37537376 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

