Fibrin Sealant TISSEEL Lyo as a haemostatic agent in vascular surgery: Results of randomized, controlled, patient-blinded, multicentre clinical study in the Russian population
- PMID: 37427979
- PMCID: PMC10358634
- DOI: 10.1177/00368504231182834
Fibrin Sealant TISSEEL Lyo as a haemostatic agent in vascular surgery: Results of randomized, controlled, patient-blinded, multicentre clinical study in the Russian population
Abstract
Background: This phase III, controlled, patient-blinded, multicentre study in two parallel, equal-sized treatment groups compared the efficacy and safety of TISSEEL Lyo, fibrin sealant versus Manual Compression (MC) with surgical gauze pads for use as a haemostatic agent in patients who underwent vascular surgery in Russia.
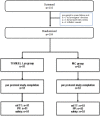
Methods: Adult patients, both genders, who received peripheral vascular expanded polytetrafluoroethylene conduits and had suture line bleeding after surgical haemostasis were enrolled. Patients were randomized to be treated with TISSEEL Lyo or MC. The bleeding needed additional treatment and had to be assessed as grade 1 or 2 bleeding according to the Validated Intraoperative Bleeding scale. The primary efficacy endpoint was the proportion of patients achieving haemostasis at 4 min after treatment application (T4) at the study suture line, which was maintained until the closure of the surgical wound. The secondary efficacy endpoints included the proportion of patients achieving haemostasis at 6 min (T6) and 10 min (T10) after treatment application at the study suture line, which was maintained until closure of the surgical wound, as well as the proportion of patients with intraoperative and postoperative rebleeding. Safety outcomes included incidence of adverse events (AEs), surgical site infections and graft occlusions.
Results: A total of 110 patients were screened; 104 patients were randomized: (TISSEEL Lyo: 51 [49%] patients; MC: 53 [51%] patients). T4 haemostasis was achieved in 43 (84.3%) patients in the TISSEEL Lyo group and in 11 (20.8%) patients in the MC group (p < 0.001). Significantly more patients in TISSEEL Lyo group achieved the haemostasis at T6 (relative risk (RR) of achieving haemostasis 1.74 [95% confidence interval (CI) 1.37; 2.35]) and T10 (RR 1.18 [95% CI 1.05; 1.38]) versus MC. No one had intraoperative rebleeding. Postoperative rebleeding was reported only in one patient in the MC group. No treatment-emergent serious AEs (TESAEs) related to TISSEEL Lyo/MC, TESAEs leading to withdrawal and TESAEs leading to death were reported in patients during the study.
Conclusions: Data demonstrated TISSEEL Lyo had clinically and statistically significant superiority to MC as a haemostatic agent in vascular surgery at all measured time points including 4, 6 and 10 min and had proven to be safe.
Keywords: Fibrin sealant; TISSEEL Lyo; surgery; thrombin.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Similar articles
-
Use of fibrin sealant as a hemostatic agent in expanded polytetrafluoroethylene graft placement surgery.Ann Vasc Surg. 2011 Aug;25(6):813-22. doi: 10.1016/j.avsg.2010.12.016. Epub 2011 Apr 21. Ann Vasc Surg. 2011. PMID: 21514114 Clinical Trial.
-
A prospective randomized study comparing fibrin sealant to manual compression for the treatment of anastomotic suture-hole bleeding in expanded polytetrafluoroethylene grafts.J Vasc Surg. 2012 Jul;56(1):134-41. doi: 10.1016/j.jvs.2012.01.009. Epub 2012 May 25. J Vasc Surg. 2012. PMID: 22633423 Clinical Trial.
-
A Prospective, Randomized, Multicenter Clinical Trial on the Safety and Efficacy of a Ready-to-Use Fibrin Sealant as an Adjunct to Hemostasis during Vascular Surgery.Ann Vasc Surg. 2017 Nov;45:127-137. doi: 10.1016/j.avsg.2017.06.043. Epub 2017 Jun 21. Ann Vasc Surg. 2017. PMID: 28647631 Clinical Trial.
-
Fibrin sealant (evicel® [quixil®/crosseal™]): a review of its use as supportive treatment for haemostasis in surgery.Drugs. 2011 Oct 1;71(14):1893-915. doi: 10.2165/11207700-000000000-00000. Drugs. 2011. PMID: 21942978 Review.
-
Fibrin and Thrombin Sealants in Vascular and Cardiac Surgery: A Systematic Review and Meta-analysis.Eur J Vasc Endovasc Surg. 2020 Sep;60(3):469-478. doi: 10.1016/j.ejvs.2020.05.016. Epub 2020 Jul 1. Eur J Vasc Endovasc Surg. 2020. PMID: 32620348
Cited by
-
Cotyledon-Specific Flow Evaluation of Rhesus Macaque Placental Injury Using Ferumoxytol Dynamic Contrast-Enhanced MRI.J Magn Reson Imaging. 2024 Nov;60(5):2196-2204. doi: 10.1002/jmri.29291. Epub 2024 Feb 20. J Magn Reson Imaging. 2024. PMID: 38375996
References
-
- Kjaergard HK, Fairbrother JE. Controlled clinical studies of fibrin sealant in cardiothoracic surgery – a review. Eur J Cardiothorac Surg 1996; 10: 727–733. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials