The Severity of Chronic Cough Diary (SCCD): development and content validation of a novel patient-reported outcome instrument for evaluating the symptom experience of chronic cough
- PMID: 37428359
- PMCID: PMC10333155
- DOI: 10.1186/s41687-023-00605-8
The Severity of Chronic Cough Diary (SCCD): development and content validation of a novel patient-reported outcome instrument for evaluating the symptom experience of chronic cough
Abstract
Background: Refractory chronic cough (RCC), a cough lasting longer than 8 weeks with an unexplained underlying etiology and unresponsive to conventional treatment, can have substantial effects on patients' quality of life. For assessment of the efficacy of antitussive medication in clinical trials in RCC, patient-reported outcome (PRO) instruments should be fit for purpose with appropriate content validity. Here we describe the qualitative testing of a newly developed PRO instrument: the Severity of Chronic Cough Diary (SCCD).
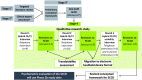
Methods: The SCCD was developed to assess patients' symptom experience of cough in patients with RCC. A preliminary version was tested and refined based on an iterative process in a qualitative study. In total, three rounds of interviews were conducted with adult participants diagnosed with RCC in the USA (n = 19) and UK (n = 10). Rounds 1-3 consisted of hybrid concept elicitation (CE) interviews and cognitive interviews (CIs), with Round 3 also including interviews in a subset of participants (n = 5) about the usability of the SCCD as administered on an electronic handheld device.
Results: The CE interviews identified concepts important to patients' experiences related to RCC that were broadly in line with the concepts in the preliminary version of the SCCD. Participants provided positive feedback on the draft SCCD across all CI rounds, reporting the instrument to be relevant and straightforward to complete, and containing a comprehensive set of concepts to evaluate their symptom experience of RCC. Participants demonstrated a good understanding of proposed item wording, response options, and the 24-hour recall period, and thought completion of the SCCD on the electronic device was easy. Following revisions based on results from each interview round, the SCCD at the end of this qualitative research study had 14 items assessing the concepts of: cough symptoms (five items), symptoms related to cough (four items), disruption to activities due to cough (three items), and disruption to sleep due to cough (two items).
Conclusions: The results of this study provide qualitative evidence supporting the content validity of the SCCD as a PRO instrument for evaluating outcomes of therapies for RCC in clinical trials.
Keywords: Content validity; Diary; PRO; Patient-reported outcome; Qualitative; RCC; Refractory chronic cough; Refractory unexplained chronic cough; Severity of Chronic Cough Diary.
© 2023. The Author(s).
Conflict of interest statement
MdlOA and CH are employees of Bayer AG. HK and AS are employees of Evidera, which was contracted by Bayer AG to conduct the study. AH was an employee of Evidera at the time of the study and is now an employee of UCB.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

