Granulocyte-colony stimulating factor ameliorates di-ethylhexyl phthalate-induced cardiac muscle injury via stem cells recruitment, Desmin protein regulation, antifibrotic and antiapoptotic mechanisms
- PMID: 37428366
- PMCID: PMC10412672
- DOI: 10.1007/s10735-023-10137-6
Granulocyte-colony stimulating factor ameliorates di-ethylhexyl phthalate-induced cardiac muscle injury via stem cells recruitment, Desmin protein regulation, antifibrotic and antiapoptotic mechanisms
Abstract
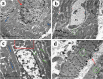
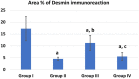
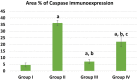
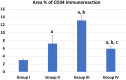
Phthalates are common plasticizers present in medical-grade plastics and other everyday products. Di-ethylhexyl phthalate (DEHP) has been noted as a causative risk factor for the initiation and augmentation of cardiovascular functional disorders. G-CSF is a glycoprotein found in numerous tissues throughout the body and is currently applied in clinical practice and has been tested in congestive heart failure. We aimed to examine in depth the effect of DEHP on the histological and biochemical structure of the cardiac muscle in adult male albino rats and the mechanisms underlying the possible ameliorative effect of G-CSF. Forty-eight adult male albino rats were divided into control group, DEHP group, DEHP+ G-CSF group and DEHP-recovery group. We measured serum levels of aspartate aminotransferase (AST), creatine kinase MB isoenzyme (CK-MB) and lactate dehydrogenase (LDH). Left ventricular sections were processed for light and electron microscope examination, and immunohistochemical staining of Desmin, activated Caspase-3 and CD34. DEHP significantly increased enzyme levels, markedly distorted the normal architecture of cardiac muscle fibers, downregulated Desmin protein levels and enhanced fibrosis, and apoptosis. G-CSF treatment significantly decreased the enzyme levels compared to DEHP group. It enhanced CD34 positive stem cells recruitment to injured cardiac muscle, therefore improved the ultrastructural features of most cardiac muscle fibers via anti-fibrotic and anti-apoptotic effects in addition to increased Desmin protein expression levels. The recovery group showed partial improvement due to persistent DEHP effect. In conclusion, administration of G-CSF effectively corrected the histopathological, immunohistochemical and biochemical alterations in the cardiac muscle after DEHP administration by stem cells recruitment, Desmin protein regulation, antifibrotic and antiapoptotic mechanisms.
Keywords: Cardiac muscle; Di-ethylhexyl phthalate; G-CSF; Histology; Immunohistochemistry.
© 2023. The Author(s).
Conflict of interest statement
There is no conflict of interests.
Figures








References
-
- Abdel Mohsen AF, Ahmed NAW, Altaib ZM, Zaher SM. Effect of cisplatin on cerebellar cortex of albino rat and possible protective role of granulocyte colony stimulating factor versus Citrullus lanatus juice: a histological Study. Egypt J Histol. 2020;43(3):702–717.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

