Initiation Patterns of Disease-Modifying Therapies for Multiple Sclerosis Among US Adults and Children, 2001 Through 2020
- PMID: 37428482
- PMCID: PMC10334299
- DOI: 10.1001/jamaneurol.2023.2125
Initiation Patterns of Disease-Modifying Therapies for Multiple Sclerosis Among US Adults and Children, 2001 Through 2020
Abstract
Importance: Many disease-modifying therapies (DMTs) have been approved for multiple sclerosis (MS) in the past 2 decades. Research evaluating how these approvals have changed real-world prescribing patterns is scarce.
Objective: To evaluate patterns in DMT initiations between 2001 and 2020 among commercially insured US adults and children with MS.
Design, setting, and participants: This serial cross-sectional study was conducted from 2001 through 2020 (mean patient enrollment duration, 4.8 years) and used US commercial claims data (MarketScan). Analysis took place between January 2022 and March 2023. Of 287 084 patients with MS identified, 113 583 patients (113 095 adults and 488 children) with MS newly initiated at least 1 DMT.
Exposure: New initiation episode of a DMT, defined as no claim for the same DMT in the previous year.
Main outcome measure: The proportion of total DMT initiations per year attributable to each DMT. Trends in initiations were evaluated annually.
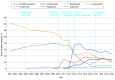
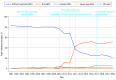
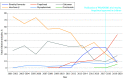
Results: The study team identified 153 846 DMT initiation episodes among adults (median age, 46 [IQR, 38-53) years]; 86 133 female [76.2%]) and 583 among children (median age, 16 (IQR, 14-17) years; 346 female [70.9%]). Among adults, use of platform injectables showed an absolute decline of 73.8% over the study period, driven by a 61.2% reduction in interferon β initiations (P < .001 for trend). In contrast, the 2010 introduction of oral DMTs led to a rise in their use from 1.1% (2010) to 62.3% (2020) of all DMT initiations (P = .002 for trend). Infusion therapy initiations remained relatively low, accounting for 3.2% of all initiations since their introduction in 2004 but increased modestly annually after ocrelizumab was introduced (2017), reaching 8.2% of all initiations in 2020 (P < .001 for trend). Children showed similar initiation patterns, except for preferred oral therapy. Between 2019 and 2020, dimethyl fumarate was the most commonly initiated DMT in adults (23.3% to 27.2% of all initiations), while in children fingolimod was the most commonly initiated (34.8% to 68.8%).
Conclusions and relevance: Current MS treatment guidelines emphasize shared decision-making between patients and clinicians to balance treatment efficacy, safety, cost, and convenience. This study found that oral DMTs were the predominant DMT type initiated by 2020. The cause of this shift cannot be determined from this study, but may reflect several factors, including convenience of administration, direct-to-consumer advertising, or insurance restrictions.
Conflict of interest statement
Figures

References
-
- National Multiple Sclerosis Society . Disease-modifying therapies for MS. Accessed May 31, 2023. https://www.nationalmssociety.org/For-Professionals/Clinical-Care/Managi...
-
- Cohen JA, Comi G, Selmaj KW, et al. ; RADIANCE Trial Investigators . Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 2019;18(11):1021-1033. doi: 10.1016/S1474-4422(19)30238-8 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

