Safety and Efficacy of Tenecteplase Compared With Alteplase in Patients With Large Vessel Occlusion Stroke: A Prespecified Secondary Analysis of the ACT Randomized Clinical Trial
- PMID: 37428494
- PMCID: PMC10334294
- DOI: 10.1001/jamaneurol.2023.2094
Safety and Efficacy of Tenecteplase Compared With Alteplase in Patients With Large Vessel Occlusion Stroke: A Prespecified Secondary Analysis of the ACT Randomized Clinical Trial
Abstract
Importance: It is unknown whether intravenous thrombolysis using tenecteplase is noninferior or preferable compared with alteplase for patients with acute ischemic stroke.
Objective: To examine the safety and efficacy of tenecteplase compared to alteplase among patients with large vessel occlusion (LVO) stroke.
Design, setting, and participants: This was a prespecified analysis of the Intravenous Tenecteplase Compared With Alteplase for Acute Ischaemic Stroke in Canada (ACT) randomized clinical trial that enrolled patients from 22 primary and comprehensive stroke centers across Canada between December 10, 2019, and January 25, 2022. Patients 18 years and older with a disabling ischemic stroke within 4.5 hours of symptom onset were randomly assigned (1:1) to either intravenous tenecteplase or alteplase and were monitored for up to 120 days. Patients with baseline intracranial internal carotid artery (ICA), M1-middle cerebral artery (MCA), M2-MCA, and basilar occlusions were included in this analysis. A total of 1600 patients were enrolled, and 23 withdrew consent.
Exposures: Intravenous tenecteplase (0.25 mg/kg) vs intravenous alteplase (0.9 mg/kg).
Main outcomes and measures: The primary outcome was the proportion of modified Rankin scale (mRS) score 0-1 at 90 days. Secondary outcomes were an mRS score from 0 to 2, mortality, and symptomatic intracerebral hemorrhage. Angiographic outcomes were successful reperfusion (extended Thrombolysis in Cerebral Infarction scale score 2b-3) on first and final angiographic acquisitions. Multivariable analyses (adjusting for age, sex, National Institute of Health Stroke Scale score, onset-to-needle time, and occlusion location) were carried out.
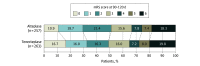
Results: Among 1577 patients, 520 (33.0%) had LVO (median [IQR] age, 74 [64-83] years; 283 [54.4%] women): 135 (26.0%) with ICA occlusion, 237 (45.6%) with M1-MCA, 117 (22.5%) with M2-MCA, and 31 (6.0%) with basilar occlusions. The primary outcome (mRS score 0-1) was achieved in 86 participants (32.7%) in the tenecteplase group vs 76 (29.6%) in the alteplase group. Rates of mRS 0-2 (129 [49.0%] vs 131 [51.0%]), symptomatic intracerebral hemorrhage (16 [6.1%] vs 11 [4.3%]), and mortality (19.9% vs 18.1%) were similar in the tenecteplase and alteplase groups, respectively. No difference was noted in successful reperfusion rates in the first (19 [9.2%] vs 21 [10.5%]) and final angiogram (174 [84.5%] vs 177 [88.9%]) among 405 patients who underwent thrombectomy.
Conclusions and relevance: The findings in this study indicate that intravenous tenecteplase conferred similar reperfusion, safety, and functional outcomes compared to alteplase among patients with LVO.
Conflict of interest statement
Figures

References
-
- Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists’ Collaborative Group . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. doi:10.1016/S0140-6736(14)60584-5 - DOI - PMC - PubMed
-
- Powers WJ, Rabinstein AA, Ackerson T, et al. . Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211 - DOI - PubMed
-
- Menon BK, Buck BH, Singh N, et al. ; AcT Trial Investigators . Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, non-inferiority trial. Lancet. 2022;400(10347):161-169. doi:10.1016/S0140-6736(22)01054-6 - DOI - PubMed
-
- Abuelazm M, Seri AR, Awad AK, et al. . The efficacy and safety of tenecteplase versus alteplase for acute ischemic stroke: an updated systematic review, pairwise, and network meta-analysis of randomized controlled trials. J Thromb Thrombolysis. 2023;55(2):322-338. doi:10.1007/s11239-022-02730-5 - DOI - PMC - PubMed
-
- Menon BK, Al-Ajlan FS, Najm M, et al. ; INTERRSeCT Study Investigators . Association of clinical, imaging, and thrombus characteristics with recanalization of visible intracranial occlusion in patients with acute ischemic stroke. JAMA. 2018;320(10):1017-1026. doi:10.1001/jama.2018.12498 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

