Effectiveness of Bundled Hyperpolypharmacy Deprescribing Compared With Usual Care Among Older Adults: A Randomized Clinical Trial
- PMID: 37428504
- PMCID: PMC10334220
- DOI: 10.1001/jamanetworkopen.2023.22505
Effectiveness of Bundled Hyperpolypharmacy Deprescribing Compared With Usual Care Among Older Adults: A Randomized Clinical Trial
Abstract
Importance: Older patients using many prescription drugs (hyperpolypharmacy) may be at increased risk of adverse drug effects.
Objective: To test the effectiveness and safety of a quality intervention intended to reduce hyperpolypharmacy.
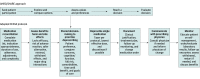
Design, setting, and participants: This randomized clinical trial allocated patients 76 years or older who used 10 or more prescription medications to a deprescribing intervention or to usual care (1:1 ratio) at an integrated health system with multiple preexisting deprescribing workflows. Data were collected from October 15, 2020, to July 29, 2022.
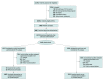
Intervention: Physician-pharmacist collaborative drug therapy management, standard-of-care practice recommendations, shared decision-making, and deprescribing protocols administered by telephone over multiple cycles for a maximum of 180 days after allocation.
Main outcomes and measures: Primary end points were change in the number of medications and in the prevalence of geriatric syndrome (falls, cognition, urinary incontinence, and pain) from 181 to 365 days after allocation compared with before randomization. Secondary outcomes were use of medical services and adverse drug withdrawal effects.
Results: Of a random sample of 2860 patients selected for potential enrollment, 2470 (86.4%) remained eligible after physician authorization, with 1237 randomized to the intervention and 1233 to usual care. A total of 1062 intervention patients (85.9%) were reached and agreed to enroll. Demographic variables were balanced. The median age of the 2470 patients was 80 (range, 76-104) years, and 1273 (51.5%) were women. In terms of race and ethnicity, 185 patients (7.5%) were African American, 234 (9.5%) were Asian or Pacific Islander, 220 (8.9%) were Hispanic, 1574 (63.7%) were White (63.7%), and 257 (10.4%) were of other (including American Indian or Alaska Native, Native Hawaiian, or >1 race or ethnicity) or unknown race or ethnicity. During follow-up, both the intervention and usual care groups had slight reductions in the number of medications dispensed (mean changes, -0.4 [95% CI, -0.6 to -0.2] and -0.4 [95% CI, -0.6 to -0.3], respectively), with no difference between the groups (P = .71). There were no significant changes in the prevalence of a geriatric condition in the usual care and intervention groups at the end of follow-up and no difference between the groups (baseline prevalence: 47.7% [95% CI, 44.9%-50.5%] vs 42.9% [95% CI, 40.1%-45.7%], respectively; difference-in-differences, 1.0 [95% CI, -3.5 to 5.6]; P = .65). No differences in use of medical services or adverse drug withdrawal effects were observed.
Conclusions and relevance: In this randomized clinical trial from an integrated care setting with various preexisting deprescribing workflows, a bundled hyperpolypharmacy deprescribing intervention was not associated with reduction in medication dispensing, prevalence of geriatric syndrome, utilization of medical services, or adverse drug withdrawal effects. Additional research is needed in less integrated settings and in more targeted populations.
Trial registration: ClinicalTrials.gov Identifier: NCT05616689.
Conflict of interest statement
Figures
Similar articles
-
The MedSafer Study-Electronic Decision Support for Deprescribing in Hospitalized Older Adults: A Cluster Randomized Clinical Trial.JAMA Intern Med. 2022 Mar 1;182(3):265-273. doi: 10.1001/jamainternmed.2021.7429. JAMA Intern Med. 2022. PMID: 35040926 Free PMC article. Clinical Trial.
-
Reducing Central Nervous System-Active Medications to Prevent Falls and Injuries Among Older Adults: A Cluster Randomized Clinical Trial.JAMA Netw Open. 2024 Jul 1;7(7):e2424234. doi: 10.1001/jamanetworkopen.2024.24234. JAMA Netw Open. 2024. PMID: 39052289 Free PMC article. Clinical Trial.
-
Deprescribing Medications Among Older Adults From End of Hospitalization Through Postacute Care: A Shed-MEDS Randomized Clinical Trial.JAMA Intern Med. 2023 Mar 1;183(3):223-231. doi: 10.1001/jamainternmed.2022.6545. JAMA Intern Med. 2023. PMID: 36745422 Free PMC article. Clinical Trial.
-
A systematic review of randomised-controlled trials on deprescribing outcomes in older adults with polypharmacy.Int J Pharm Pract. 2023 Jun 30;31(4):349-368. doi: 10.1093/ijpp/riad025. Int J Pharm Pract. 2023. PMID: 37155330
-
Deprescribing fall-risk increasing drugs (FRIDs) for the prevention of falls and fall-related complications: a systematic review and meta-analysis.BMJ Open. 2021 Feb 10;11(2):e035978. doi: 10.1136/bmjopen-2019-035978. BMJ Open. 2021. PMID: 33568364 Free PMC article.
Cited by
-
Associations between sex, race/ethnicity, and age and the initiation of chronic high-risk medication in US older adults.J Am Geriatr Soc. 2024 Dec;72(12):3705-3718. doi: 10.1111/jgs.19173. Epub 2024 Aug 31. J Am Geriatr Soc. 2024. PMID: 39215549 Free PMC article.
-
Interventions to Address Potentially Inappropriate Prescribing for Older Primary Care Patients: A Systematic Review and Meta-Analysis.JAMA Netw Open. 2025 Jun 2;8(6):e2517965. doi: 10.1001/jamanetworkopen.2025.17965. JAMA Netw Open. 2025. PMID: 40577011 Free PMC article.
-
Deprescribing for people living with dementia: ALIGNing interventions and outcomes.J Am Geriatr Soc. 2024 Jul;72(7):1949-1951. doi: 10.1111/jgs.18946. Epub 2024 May 15. J Am Geriatr Soc. 2024. PMID: 38751095 Free PMC article. No abstract available.
-
Medication Optimization Protocol Efficacy for Geriatric Inpatients: A Randomized Clinical Trial.JAMA Netw Open. 2024 Jul 1;7(7):e2423544. doi: 10.1001/jamanetworkopen.2024.23544. JAMA Netw Open. 2024. PMID: 39078632 Free PMC article. Clinical Trial.
-
Deprescribing strategies in older patients with heart failure.Intern Emerg Med. 2025 Mar;20(2):599-609. doi: 10.1007/s11739-024-03791-5. Epub 2024 Oct 15. Intern Emerg Med. 2025. PMID: 39406965 Review.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

