Long QT syndrome-associated calmodulin variants disrupt the activity of the slowly activating delayed rectifier potassium channel
- PMID: 37428651
- PMCID: PMC10952621
- DOI: 10.1113/JP284994
Long QT syndrome-associated calmodulin variants disrupt the activity of the slowly activating delayed rectifier potassium channel
Abstract
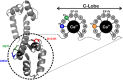
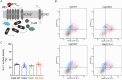
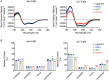
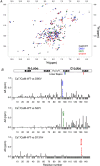
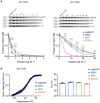
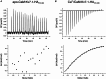
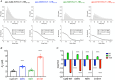
Calmodulin (CaM) is a highly conserved mediator of calcium (Ca2+ )-dependent signalling and modulates various cardiac ion channels. Genotyping has revealed several CaM mutations associated with long QT syndrome (LQTS). LQTS patients display prolonged ventricular recovery times (QT interval), increasing their risk of incurring life-threatening arrhythmic events. Loss-of-function mutations to Kv7.1 (which drives the slow delayed rectifier potassium current, IKs, a key ventricular repolarising current) are the largest contributor to congenital LQTS (>50% of cases). CaM modulates Kv7.1 to produce a Ca2+ -sensitive IKs, but little is known about the consequences of LQTS-associated CaM mutations on Kv7.1 function. Here, we present novel data characterising the biophysical and modulatory properties of three LQTS-associated CaM variants (D95V, N97I and D131H). We showed that mutations induced structural alterations in CaM and reduced affinity for Kv7.1, when compared with wild-type (WT). Using HEK293T cells expressing Kv7.1 channel subunits (KCNQ1/KCNE1) and patch-clamp electrophysiology, we demonstrated that LQTS-associated CaM variants reduced current density at systolic Ca2+ concentrations (1 μm), revealing a direct QT-prolonging modulatory effect. Our data highlight for the first time that LQTS-associated perturbations to CaM's structure impede complex formation with Kv7.1 and subsequently result in reduced IKs. This provides a novel mechanistic insight into how the perturbed structure-function relationship of CaM variants contributes to the LQTS phenotype. KEY POINTS: Calmodulin (CaM) is a ubiquitous, highly conserved calcium (Ca2+ ) sensor playing a key role in cardiac muscle contraction. Genotyping has revealed several CaM mutations associated with long QT syndrome (LQTS), a life-threatening cardiac arrhythmia syndrome. LQTS-associated CaM variants (D95V, N97I and D131H) induced structural alterations, altered binding to Kv7.1 and reduced IKs. Our data provide a novel mechanistic insight into how the perturbed structure-function relationship of CaM variants contributes to the LQTS phenotype.
Keywords: IKs; Kv7.1; LQTS; calcium; calmodulin; cardiac arrhythmia.
© 2023 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Comment in
-
Calmodulin mutations can underlie the phenotype of long QT syndrome variant 1.J Physiol. 2023 Sep;601(17):3695-3696. doi: 10.1113/JP285220. Epub 2023 Aug 9. J Physiol. 2023. PMID: 37555447 No abstract available.
References
-
- Adam, M. P. , Mirzaa, G. M. , Pagon, R. A. , Wallace, S. E. , Bean, L. J. H. , Gripp, K. W. , & Amemiya, A. (1993). GeneReviews. Advance online publication. https://www.ncbi.nlm.nih.gov/pubmed/20301308
-
- Asada, K. , Kurokawa, J. , & Furukawa, T. (2009). Redox‐ and calmodulin‐dependent S‐nitrosylation of the KCNQ1 channel. Journal of Biological Chemistry, 284(9), 6014–6020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

