Image-based flow simulation of platelet aggregates under different shear rates
- PMID: 37428797
- PMCID: PMC10358939
- DOI: 10.1371/journal.pcbi.1010965
Image-based flow simulation of platelet aggregates under different shear rates
Abstract
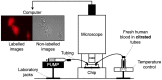
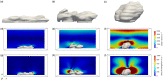
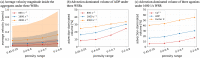
Hemodynamics is crucial for the activation and aggregation of platelets in response to flow-induced shear. In this paper, a novel image-based computational model simulating blood flow through and around platelet aggregates is presented. The microstructure of aggregates was captured by two different modalities of microscopy images of in vitro whole blood perfusion experiments in microfluidic chambers coated with collagen. One set of images captured the geometry of the aggregate outline, while the other employed platelet labelling to infer the internal density. The platelet aggregates were modelled as a porous medium, the permeability of which was calculated with the Kozeny-Carman equation. The computational model was subsequently applied to study hemodynamics inside and around the platelet aggregates. The blood flow velocity, shear stress and kinetic force exerted on the aggregates were investigated and compared under 800 s-1, 1600 s-1 and 4000 s-1 wall shear rates. The advection-diffusion balance of agonist transport inside the platelet aggregates was also evaluated by local Péclet number. The findings show that the transport of agonists is not only affected by the shear rate but also significantly influenced by the microstructure of the aggregates. Moreover, large kinetic forces were found at the transition zone from shell to core of the aggregates, which could contribute to identifying the boundary between the shell and the core. The shear rate and the rate of elongation flow were investigated as well. The results imply that the emerging shapes of aggregates are highly correlated to the shear rate and the rate of elongation. The framework provides a way to incorporate the internal microstructure of the aggregates into the computational model and yields a better understanding of the hemodynamics and physiology of platelet aggregates, hence laying the foundation for predicting aggregation and deformation under different flow conditions.
Copyright: © 2023 Hao et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures










Similar articles
-
Analysis of morphology of platelet aggregates formed on collagen under laminar blood flow.Ann Biomed Eng. 2011 Feb;39(2):922-9. doi: 10.1007/s10439-010-0182-4. Epub 2010 Oct 15. Ann Biomed Eng. 2011. PMID: 20949319
-
Hydrodynamic effects and receptor interactions of platelets and their aggregates in linear shear flow.Biophys J. 1997 Nov;73(5):2819-35. doi: 10.1016/S0006-3495(97)78311-5. Biophys J. 1997. PMID: 9370476 Free PMC article.
-
Continuum modeling of thrombus formation and growth under different shear rates.J Biomech. 2022 Feb;132:110915. doi: 10.1016/j.jbiomech.2021.110915. Epub 2022 Jan 4. J Biomech. 2022. PMID: 35032838
-
Mathematical analysis of mural thrombogenesis. Concentration profiles of platelet-activating agents and effects of viscous shear flow.Biophys J. 1989 Dec;56(6):1121-41. doi: 10.1016/S0006-3495(89)82760-2. Biophys J. 1989. PMID: 2611327 Free PMC article.
-
The function of ultra-large von Willebrand factor multimers in high shear flow controlled by ADAMTS13.Hamostaseologie. 2015;35(3):225-33. doi: 10.5482/HAMO-14-12-0077. Epub 2015 May 18. Hamostaseologie. 2015. PMID: 25983111 Review.
Cited by
-
The impact of clot permeability on platelet fluxes toward its surface.PLoS One. 2025 Mar 25;20(3):e0317828. doi: 10.1371/journal.pone.0317828. eCollection 2025. PLoS One. 2025. PMID: 40132156 Free PMC article.
References
-
- Hou Y, Carrim N, Wang Y, Gallant RC, Marshall A, Ni H. Platelets in hemostasis and thrombosis: Novel mechanisms of fibrinogen-independent platelet aggregation and fibronectinmediated protein wave of hemostasis. The Journal of Biomedical Research. 2015;29(jbr150602):437. doi: 10.7555/JBR.29.20150121 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

