Interventions affecting the nitric oxide pathway versus placebo or no therapy for fetal growth restriction in pregnancy
- PMID: 37428872
- PMCID: PMC10332237
- DOI: 10.1002/14651858.CD014498
Interventions affecting the nitric oxide pathway versus placebo or no therapy for fetal growth restriction in pregnancy
Abstract
Background: Fetal growth restriction (FGR) is a condition of poor growth of the fetus in utero. One of the causes of FGR is placental insufficiency. Severe early-onset FGR at < 32 weeks of gestation occurs in an estimated 0.4% of pregnancies. This extreme phenotype is associated with a high risk of fetal death, neonatal mortality, and neonatal morbidity. Currently, there is no causal treatment, and management is focused on indicated preterm birth to prevent fetal death. Interest has risen in interventions that aim to improve placental function by administration of pharmacological agents affecting the nitric oxide pathway causing vasodilatation.
Objectives: The objective of this systematic review and aggregate data meta-analysis is to assess the beneficial and harmful effects of interventions affecting the nitric oxide pathway compared with placebo, no therapy, or different drugs affecting this pathway against each other, in pregnant women with severe early-onset FGR.
Search methods: We searched Cochrane Pregnancy and Childbirth's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (16 July 2022), and reference lists of retrieved studies.
Selection criteria: We considered all randomised controlled comparisons of interventions affecting the nitric oxide pathway compared with placebo, no therapy, or another drug affecting this pathway in pregnant women with severe early-onset FGR of placental origin, for inclusion in this review.
Data collection and analysis: We used standard Cochrane Pregnancy and Childbirth methods for data collection and analysis.
Main results: We included a total of eight studies (679 women) in this review, all of which contributed to the data and analysis. The identified studies report on five different comparisons: sildenafil compared with placebo or no therapy, tadalafil compared with placebo or no therapy, L-arginine compared with placebo or no therapy, nitroglycerin compared with placebo or no therapy and sildenafil compared with nitroglycerin. The risk of bias of included studies was judged as low or unclear. In two studies the intervention was not blinded. The certainty of evidence for our primary outcomes was judged as moderate for the intervention sildenafil and low for tadalafil and nitroglycerine (due to low number of participants and low number of events). For the intervention L-arginine, our primary outcomes were not reported. Sildenafil citrate compared to placebo or no therapy (5 studies, 516 women) Five studies (Canada, Australia and New Zealand, the Netherlands, the UK and Brazil) involving 516 pregnant women with FGR were included. We assessed the certainty of the evidence as moderate. Compared with placebo or no therapy, sildenafil probably has little or no effect on all-cause mortality (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.80 to 1.27, 5 studies, 516 women); may reduce fetal mortality (RR 0.82, 95% CI 0.60 to 1.12, 5 studies, 516 women), and increase neonatal mortality (RR 1.45, 95% CI 0.90 to 2.33, 5 studies, 397 women), although the results are uncertain for fetal and neonatal mortality as 95% confidence intervals are wide crossing the line of no effect. Tadalafil compared with placebo or no therapy (1 study, 87 women) One study (Japan) involving 87 pregnant women with FGR was included. We assessed the certainty of the evidence as low. Compared with placebo or no therapy, tadalafil may have little or no effect on all-cause mortality (risk ratio 0.20, 95% CI 0.02 to 1.60, one study, 87 women); fetal mortality (RR 0.11, 95% CI 0.01 to 1.96, one study, 87 women); and neonatal mortality (RR 0.89, 95% CI 0.06 to 13.70, one study, 83 women). L-Arginine compared with placebo or no therapy (1 study, 43 women) One study (France) involving 43 pregnant women with FGR was included. This study did not assess our primary outcomes. Nitroglycerin compared to placebo or no therapy (1 studies, 23 women) One study (Brazil) involving 23 pregnant women with FGR was included. We assessed the certainty of the evidence as low. The effect on the primary outcomes is not estimable due to no events in women participating in both groups. Sildenafil citrate compared to nitroglycerin (1 study, 23 women) One study (Brazil) involving 23 pregnant women with FGR was included. We assessed the certainty of the evidence as low. The effect on the primary outcomes is not estimable due to no events in women participating in both groups.
Authors' conclusions: Interventions affecting the nitric oxide pathway probably do not seem to influence all-cause (fetal and neonatal) mortality in pregnant women carrying a baby with FGR, although more evidence is needed. The certainty of this evidence is moderate for sildenafil and low for tadalafil and nitroglycerin. For sildenafil a fair amount of data are available from randomised clinical trials, but with low numbers of participants. Therefore, the certainty of evidence is moderate. For the other interventions investigated in this review there are insufficient data, meaning we do not know whether these interventions improve perinatal and maternal outcomes in pregnant women with FGR.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Anouk Pels and Wessel Ganzevoort performed the assessment and data extraction of the studiesSharp 2018, Groom 2019 and von Dadelszen 2022. Aris Papageorghiou and Katie Groom performed the assessment and data extraction of Pels 2020.
Anouk Pels: was involved in the Dutch STRIDER trial, a randomised controlled trial investigating sildenafil versus placebo in severe, early‐onset fetal growth restriction. The Dutch STRIDER trial was eligible for inclusion in the systematic review. She was not involved in any decisions relating to the study (inclusion/exclusion, risk of bias, data extraction, GRADE) and these tasks were carried out by other members of the review team who were not directly involved in the Dutch STRIDER trial.
Wessel Ganzevoort: has received government support for the conduct of investigator‐initiated clinical trials (Dutch STRIDER trial fund by ZonMW). He has also received free of charge supply of testing materials from Roche Diagnostics in clinical studies that focus on placental insufficiency. He reports not being involved in any decisions relating to the study (inclusion/exclusion, risk of bias, data extraction, GRADE) and these tasks were carried out by other members of the review team who were not directly involved in the Dutch STRIDER trial.
Louise C Kenny is Executive Pro‐Vice Chancellor of the Faculty of Health and Life Sciences at the University of Liverpool and Professor of Maternal and Fetal Health and as such has numerous grant applications under review at any given time. Anouk Pels: has been paid by Alere to give invited symposia on a proprietary screening test for preeclampsia. She is the editor of Ten Teachers and has received royalties from the publishers. She is also a limited shareholder in Metabolomic Diagnostics, an SME who have licensed technology that she has developed pertaining to the screening of preeclampsia. She is a co‐investigator for the UK STRIDER trial, funded by the National Institute for Health Research and Medical Research Council. She reports not being involved in any decisions relating to the study (inclusion/exclusion, risk of bias, data extraction, GRADE) and these tasks were carried out by other members of the review team who were not directly involved in the UK STRIDER trial.
Philip N Baker: received Health Research Council (NZ) funding to his previous institution (university of Auckland) for a randomised controlled trial of sildenafil therapy in severe, early onset intrauterine growth restriction. He is a minority shareholder in Metabolomic Diagnostics, which seeks to develop screening tests for major pregnancy complications, including fetal growth restriction. He also holds a patent (no monies received) for the diagnosis of pre‐eclampsia/fetal growth restriction (No. PCT/EP2010/070446). The patent application includes 14 metabolites as individual prognostic variables of pre‐eclampsia, and makes claims to combinations of key metabolites, which are likely to be employed in a prognostic product. He is a co‐investigator on the UK, NZ/Australia and Canadian STRIDER trials. He reports not being involved in any decisions relating to the study (inclusion/exclusion, risk of bias, data extraction, GRADE) and these tasks were carried out by other members of the review team who were not directly involved in the UK and NZ/Australia STRIDER trials.
Peter von Dadelszen: is a co‐investigator on the UK and Canadian STRIDER trials. He reports not being involved in any decisions relating to the studies (inclusion/exclusion, risk of bias, data extraction, GRADE) and these tasks were carried out by other members of the review team who were not directly involved in the UK and Canadian STRIDER trials.
Christian Gluud: is the Co‐ordinating Editor of the Cochrane Hepato‐Biliary Group and is a co‐investigator on the Dutch STRIDER trial. He reports not being involved in any decisions relating to the studies (inclusion/exclusion, risk of bias, data extraction, GRADE) and these tasks were carried out by other members of the review team who were not directly involved in the Dutch STRIDER trial.
Chirag T Kariya: CTK is is a co‐investigator for the Canadian STRIDER trial, funded by Canadian Institute of Health Research (CIHR) grant to the University of British Columbia that included support to travel to meetings for the study, conducting study related activities and salary support. They report not being involved in any decisions relating to the study (inclusion/exclusion, risk of bias, data extraction, GRADE) and these tasks were carried out by other members of the review team who were not directly involved in the Canadian STRIDER trial.
Aleid G Leemhuis: was involved in the Dutch STRIDER trial, a randomised controlled trial investigating sildenafil versus placebo in severe, early‐onset fetal growth restriction. The Dutch STRIDER trial was eligible for inclusion in the systematic review. She was not involved in any decisions relating to the study (inclusion/exclusion, risk of bias, data extraction, GRADE) and these tasks were carried out by other members of the review team who were not directly involved in the Dutch STRIDER trial. Aleid is a Pediatrician and senior investigator in the Deparrment of Neonatology Amsterdam UMC.
Katie M Groom: I am an employee of the University of Auckland. I have received travel/accommodation/meeting expenses in my role as board member for the Australian Clinical Trials Alliance (2016‐2018). I am the lead author of the STRIDER New Zealand/Australia trial.I was not involved in any decisions relating to the study (inclusion/exclusion, risk of bias, data extraction, GRADE) and these tasks were carried out by other members of the review team who were not directly involved in the New Zealand/Australia STRIDER trial.
Andrew N Sharp: Andrew Sharp is a co‐applicant on a NIHR EME funded randomised controlled trial of the effect of sildenafil on fetal growth in severe early‐onset IUGR (UK STRIDER). The results of this RCT are available for inclusion in this review. He reports not being involved in any decisions relating to the study (inclusion/exclusion, risk of bias, data extraction, GRADE) and these tasks were carried out by other members of the review team who were not directly involved in the UK STRIDER trial.
Laura Magee: Consultancy: Legal work for the Canadian Medical Protective Associate in reviewing cases of colleagues. Management of hypertension is the area that I am most frequently asked to comment on. Employment: King’s College London. Obstetric physician and Professor of Women's Health Grants/grants pending: NIHR grant as CI. Other grants as co‐investigator (UKRI, Wellcome). WILL Trial PRECISE, PRECISE‐DYAD. Co‐investigator for Canadian STRIDER trial funded by Canadian Institute of Health Research (CIHR). She reports not being involved in any decisions relating to the study (inclusion/exclusion, risk of bias, data extraction, GRADE) and these tasks were carried out by other members of the review team who were not directly involved in the Canadian STRIDER trial.
Janus C Jakobsen: is a contractor on the STRIDER consortium, but declares no other conflicts.
Ben Willem J Mol has received payment for consultancy from ObsEva Geneva (member of an advisory board since 2013), Guerbet (individual advice on Lipiodol), Merck (advisory activities) and IGenomix (member of advisory board since 2019). He has received payment for review preparation from the European Journal of Obstetrics and Gynaecology and has received travel/accommodation/meeting expenses for various non‐commercial scientific meetings (ASRM/ESHRE) as invited speaker. He has also received payment for expert testimony (Cicil cases in Australia). He is supported by a NHMRC Investigatorgrant (GNT1176437). He reports consultancy for ObsEva and Merck and travel support from Merck. He is an Editor for Cochrane Pregnancy and Childbirth, but was not involved in the editorial process for this review. He was involved in the organization of the STRIDER consortium, but was not involved as an author in the individual trials.
Aris T Papageorghiou: I am employed as a consultant in the NHS; and as an academic at the University. I also provide obstetric ultrasound in the private health sector. My research is mostly funded by public bodies, research councils, foundations and charities in the UK, Europe and USA (currently: HTA/NIHR; EPSRC/NIHR; RCUK/GCRF; NIHR/BRC; ERC; NIH; Bill & Melinda Gates Foundation). The NIHR funded the Maternal sildenafil for severe fetal growth restriction (STRIDER) trial, and this Cochrane review was conceived and planned during a STRIDER meeting, but not funded directly. I have previously participated in research where companies contributed to a grant (GE, Philips, Samsung, Premaitha Health). As an academic I often participate in research meetings at other hospitals or universities; these are unpaid but travel to attend meetings is usually provided. Some of these were sponsored by industry to the host institutions (Roche, Philips, Samsung, GE). I participate as a speaker in educational events that have, on occasion, received industrial sponsorship to cover travel, accomodation and honoraria. I receive royalties for medical text‐books I have published (Informa Healthcare, Oxford University Press). I am a co‐founder, shareholder, and senior scientific advisor for Intelligent Ultrasound, a company that aims to improve clinical ultrasound. For this I receive payments, managed through Oxford University Innovations, a subsidiary of the University of Oxford that manages technology transfer and academic consulting activities. Finally, I am a Editor‐in‐Chief for BJOG, for which I am paid a stipend; Visiting Professor at Beijing Capital University (not remunerated); a board member of the journal Ultrasound in Obstetrics and Gynecology (not remunerated); and Chair of the Expert Working Group, Obs & Gyn, Health & Social Care Information Centre (not remunerated). He reports not being involved in any decisions relating to the study (inclusion/exclusion, risk of bias, data extraction, GRADE) and these tasks were carried out by other members of the review team who were not directly involved in the UK STRIDER trial.
Figures
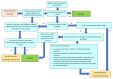
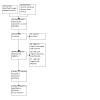

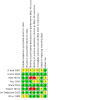












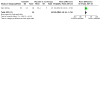
























































References
References to studies included in this review
Di Iorio 2002 {published data only}
-
- Di Iorio R, Marinoni E, Gazzolo D, Letizia C, Di Netta T, Cosmi EV. Maternal nitric oxide supplementation increases adrenomedullin concentrations in growth retarded fetuses. Gynecological Endocrinology 2002;16(3):187-92. [CENTRAL: CN-00409615] [EMBASE: 2002281231] [PMID: ] - PubMed
Groom 2019 {published data only}
-
- ACTRN12612000584831. STRIDER (NZAus): a randomised placebo-controlled trial of a new therapy (sildenafil) to help growth in severely growth restricted fetuses at very early gestations [STRIDER (NZAus): A randomised placebo controlled trial of sildenafil therapy to improve fetal growth velocity in dismal prognosis early-onset intrauterine growth restriction (New Zealand and Australia)]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12612000584831 (first received 2012). [CENTRAL: CN-01829968]
-
- Groom KM, McCowan LM, Mackay LK, Lee AC, Gardener G, Unterscheider J, et al. STRIDER NZAus: a multicentre randomised controlled trial of sildenafil therapy in early-onset fetal growth restriction. BJOG: an international journal of obstetrics and gynaecology 2019;126(8):997-1006. [CENTRAL: CN-01943789] [EMBASE: 626815376] [PMID: ] - PubMed
-
- Harris S, McKinlay C, Groom K, Beker F, Kochar A, Gill A. Sildenafil for severe, early-onset intrauterine growth restriction and neonatal cardiovascular function: a substudy of the strider NZAus randomised placebo-controlled trial. Journal of Paediatrics and Child Health 2019;55(Suppl 1):23. [CENTRAL: CN-01938831] [EMBASE: 627192292] - PMC - PubMed
-
- Harris SL, McKinlay C, Groom K, Beker F, Kochar A, Gill A. Neonatal cardiovascular function after antenatal sildenafil for severe, early-onset intrauterine growth restriction: a substudy of the STRIDER NZAus randomized placebo-controlled trial. Journal of Pediatrics 2019;1:100009. [CENTRAL: CN-02052526] [EMBASE: 2003862028] - PMC - PubMed
Maki 2019a {published data only}
-
- Maki S, Tanaka H, Magawa S, Tsuji M, Furuhashi F, Nii M, et al. 374: tadalafil treatment for fetus with early onset growth restriction (tadafer) a multicenter phase ii trial. American Journal of Obstetrics and Gynecology 2019;220(1):S257-8. [CENTRAL: CN-01773898] [EMBASE: 2001405029]
-
- Maki S, Tanaka H, Tsuji M, Furuhashi F, Magawa S, Kaneda MK, et al. Safety evaluation of tadalafil treatment for fetuses with early-onset growth restriction (TADAFER): results from the phase II trial. Journal of Clinical Medicine 2019;8(6):856. [CENTRAL: CN-01977037] [EMBASE: 2002230227] - PMC - PubMed
Pels 2020 {published data only}
-
- EUCTR2012-004112-63-NL. Sildenafil for early severe fetal growth restriction [The Dutch STRIDER (Sildenafil TheRapy In Dismal prognosis Early-onset intrauterine growth Restriction) Trial - Dutch STRIDER]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2012-004112-63-NL (first received 2014). [CENTRAL: CN-01842313]
-
- NCT02277132. The Dutch STRIDER (Sildenafil TheRapy In Dismal Prognosis Early-onset Fetal Growth Restriction). Https://clinicaltrials.gov/show/nct02277132 (first received 2014 Sep 29). [CENTRAL: CN-01589990]
-
- NTR4751. Does Sildenafil improve the neonatal prognosis in severe early onset growth restriction? [The Dutch STRIDER (Sildenafil TheRapy In Dismal prognosis Early-onset fetal growth Restriction) - The Dutch STRIDER]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=NTR4751 (first received 2014). [CENTRAL: CN-01874095]
-
- Pels A, Derks J, Elvan-Taspinar A, Van Drongelen J, De Boer M, Duvekot H, et al. LB 2: maternal sildenafil for severe early-onset fetal growth restriction: the Dutch multicentre placebo-controlled double-blind STRIDER-trial. American Journal of Obstetrics and Gynecology 2019;220(1 Suppl):S682-3. [CENTRAL: CN-01742344] [EMBASE: 2001405177]
Sharp 2018 {published data only}
-
- EUCTR2013-005398-32-GB. STRIDER - Sildenafil therapy for treatment of intrauterine growth restriction [A randomised controlled trial of sildenafil therapy in dismal prognosis early-onset intrauterine growth restriction - STRIDER version 2.0]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2013-005398-32-GB (first received 2015). [CENTRAL: CN-01910167]
-
- ISRCTN39133303. Sildenafil therapy in dismal prognosis early-onset intrauterine growth restriction [A randomised controlled trial of sildenafil therapy in dismal prognosis early-onset intrauterine growth restriction]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN39133303 (first received 2014). [CENTRAL: CN-01861980]
-
- Khalil A, Sharp A, Cornforth C, Jackson R, Mousa H, Stock S, et al. Effect of sildenafil on maternal hemodynamics in pregnancies complicated by severe early-onset fetal growth restriction: planned subgroup analysis from a multicenter randomized placebo-controlled double-blind trial. Ultrasound in Obstetrics & Gynecology 2019;55(2):198-209. [CENTRAL: CN-01987894] [EMBASE: 629130284] [PMID: ] - PubMed
-
- Khalil A, Sharp A, Cornforth C, Jackson R, Mousa H, Stock S, et al. Maternal cardiovascular changes secondary to sildenafil intake in pregnancies complicated by severe fetal growth restriction: STRIDER trial. BJOG: an international journal of obstetrics and gynaecology 2018;125(Suppl 2):25. [CENTRAL: CN-01607684] [EMBASE: 622019719]
-
- Sharp A, Cornforth C, Jackson R, Harrold J, Turner MA, Kenny LC, et al. Maternal sildenafil for severe fetal growth restriction (STRIDER): a multicentre, randomised, placebo-controlled, double-blind trial. Lancet Child and Adolescent Health 2018;2(2):93-102. [CENTRAL: CN-01982155] [EMBASE: 620544342] [PMID: ] - PubMed
Trapani 2016a {published data only}
-
- Trapani A Jr, Goncalves LF, Trapani TF, Franco MJ, Galluzzo RN, Pires MM. Comparison between transdermal nitroglycerin and sildenafil citrate in intrauterine growth restriction: effects on uterine, umbilical and fetal middle cerebral artery pulsatility indices. Ultrasound in Obstetrics & Gynecology 2016;48(1):61-5. - PubMed
von Dadelszen 2022 {published data only}
-
- NCT02442492. Sildenafil Therapy In Dismal Prognosis Early-Onset Intrauterine Growth Restriction (STRIDERCan). https://clinicaltrials.gov/ct2/show/ (first received 2015 May 5).
-
- Dadelszen P, Audibert F, Bujold E, Bone JN, Sandhu A, Li J, et al. Halting the Canadian STRIDER randomised controlled trial of sildenafil for severe, early-onset fetal growth restriction: ethical, methodological, and pragmatic considerations. BMC Research Notes 2022;15(1):244. [CENTRAL: CN-02420483] [PMID: ] - PMC - PubMed
Winer 2009 {published data only}
-
- NCT00549575. L-arginine treatment for severe vascular fetal intrauterine growth restriction: a randomized double bind controlled trial (L arginine in IUGR) [L-Arginine Treatment for Severe Vascular Fetal Intrauterine Growth Restriction: a Randomized Double Bind Controlled Trial]. Https://clinicaltrials.gov/show/NCT00549575 (first received 2007 Oct 24). [CENTRAL: CN-02013934]
-
- Winer N, Branger B, Azria E, Roze JC, Descamps P, Philippe HJ, et al. Treatment of severe vascular fetal intrauterine growth restriction with L-arginine: a multicenter prospective double-blind randomized placebo-controlled study. Ultrasound in Obstetrics & Gynecology 2007;30:381. [CENTRAL: CN-00615736]
-
- Winer N, Branger B, Azria E, Tsatsaris V, Philippe HJ, Roze JC, et al. L-Arginine treatment for severe vascular fetal intrauterine growth restriction: a randomized double-blind controlled trial. Clinical Nutrition (Edinburgh, Scotland) 2009 Jun;28(3):243-8. [CENTRAL: CN-00706952] [EMBASE: 50473765] [PMID: ] - PubMed
References to studies excluded from this review
Babar 2018 {published data only}
-
- Babar A, Bujold E, Leblanc V, Lavoie-Lebel E, Paquette J, Bazinet L, et al. Changes in endothelial function, arterial stiffness and blood pressure in pregnant women after consumption of high-flavanol and high-theobromine chocolate: a double blind randomized clinical trial. Hypertension in Pregnancy 2018;37(2):68-80. [CENTRAL: CN-01642877] [EMBASE: 621712092] [PMID: ] - PubMed
Bowkalow 2018 {published data only}
-
- Bowkalow S, Schleussner E, Kahler C, Schneider U, Lehmann T, Groten T. Pentaerythrityltetranitrate (PETN) improves utero- and feto-placental Doppler parameters in pregnancies with impaired utero-placental perfusion in mid-gestation - a secondary analysis of the PETN-pilot trial. Journal of Perinatal Medicine 2018;46(9):1004-9. [CENTRAL: CN-01708467] [PMID: ] - PubMed
Bujold 2016 {published data only}
-
- Bujold E, Babar A, Lavoie E, Girard M, Leblanc V, Lemieux S, et al. High-flavanol chocolate to improve placental function and to decrease the risk of preeclampsia: a double blind randomized clinical trial. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S23-24, Abstract no: 32.
Camarena‐Pulido 2016 {published data only}
-
- Pulido EE, Benavides LG, Baron JG, Gonzalez SP, Saray AJ, Padilla FE, et al. Efficacy of l-arginine for preventing preeclampsia in high-risk pregnancies: a double-blind, randomized, clinical trial. Hypertension in Pregnancy 2016;35(2):217-25. [CENTRAL: CN-01166503] [EMBASE: 20160242492] [PMID: ] - PubMed
Decano 2000 {published data only}
-
- Decano MB, Cabrera LT. The effects of transdermal nitroglycerin (nitrol patch) on the uterine and umbilical artery blood flow in preeclampsia: a randomized double blind placebo controlled study [abstract]. In: XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3-8; Washington DC, USA (Book 1). 2000:26. [CENTRAL: CN-00355035]
DRKS00011374 {published data only}
-
- DRKS00011374. Pentaeritrithyl tetranitrate (PETN) for secondary prevention of intrauterine growth restriction [Pentaeritrithyl tetranitrate (PETN) for secondary prevention of intrauterine growth restriction - PETN Trial]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00011374 (first received 2017). [CENTRAL: CN-01888570]
El‐Hamedi 2001 {published data only}
-
- El-Hamedi A, Shillito TJA, Simpson NAB, Walker JJ. A prospective randomised controlled trial of nitric oxide donors in at risk pregnancy [abstract]. Journal of Obstetrics and Gynaecology 2001;21 Suppl 1:S22. [CENTRAL: CN-00363807]
El‐Sayed 2018a {published data only}
-
- El-Sayed MA, Saleh SA, Maher MA, Khidre AM. Effect of sildenafil citrate on uteroplacental perfusion Doppler indices in growth-restricted fetuses. Menoufia Medical Journal 2018;31(1):31-7. - PubMed
EUCTR2014‐003138‐18‐IE {published data only}
-
- EUCTR2014-003138-18-IE. STRIDER Ireland [STRIDER Ireland: A randomised controlled trial of sildenafil therapy In dismal prognosis early-0nset intrauterine growth restriction]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014-003138-18-IE (first received 2015). [CENTRAL: CN-01904581]
Furuhashi 2021 {published data only}
-
- Furuhashi F, Tanaka H, Maki S, Tsuji M, Magawa S, Kaneda MK, et al. Tadalafil treatment for preeclampsia (medication in preeclampsia; MIE): a multicenter phase II clinical trial. Journal of Maternal-fetal & Neonatal Medicine 2021;34(22):3709-15. [CENTRAL: CN-02006473] [EMBASE: 2003630970] [PMID: ] - PubMed
Groten 2012 {published data only}
-
- Groten T, Fitzgerald J, Lehmann T, Schneider U, Kahler C, Schleussner E. Reduction of preeclampsia related complications with with the NO-donor penterythriltetranitrat (petn) in risk pregnancies - A prospective randomized double-blind placebo pilot study. Pregnancy Hypertension 2012;2(3):181. [CENTRAL: CN-02093669] - PubMed
Groten 2019 {published data only}
-
- Groten T, Lehmann T, Schleussner E, Pecks U, Von Kaisenberg C, Condic M, et al. Does Pentaerytrithyltetranitrate reduce fetal growth restriction in pregnancies complicated by uterine mal-perfusion? Study protocol of the PETN-study: a randomized controlled multicenter-trial. BMC Pregnancy and Childbirth 2019;19(1):336. [CENTRAL: CN-01987100] [EMBASE: 629299641] [PMID: ] - PMC - PubMed
-
- Groten T, Schleussner E. Pentaerithrityl tetranitrate (PETN) for secondary prevention of intrauterine growth restriction (PETN-Trial). Placenta 2019;83:e80. [CENTRAL: CN-01996848] [EMBASE: 2002510233]
Hladunewich 2006 {published data only}
-
- Hladunewich MA, Derby GC, Lafayette RA, Blouch KL, Druzin ML, Myers BD. Effect of L-arginine therapy on the glomerular injury of preeclampsia: a randomized controlled trial. Obstetrics and Gynecology 2006;107(4):886-95. [CENTRAL: CN-00556006] [EMBASE: 2006242132] [PMID: ] - PubMed
IRCT20120215009014N419 {published data only}
-
- IRCT20120215009014N419. Effect of L-arginine versus placebo on intrauterine growth of the fetus in primigravid women. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20120215009014N419 (first received 2022). [CENTRAL: CN-02410603]
IRCT20140317017034N9 {published data only}
-
- IRCT20140317017034N9. Effect of pentoxifiyllin on fetal growth restriction [The effect of Pentoxifiylline administration on placenta circulation and pregnancy out come in Fetal Growth Restriction]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20140317017034N9 (first received 2019). [CENTRAL: CN-02069014]
jRCTs041180121 {published data only}
-
- jRCTs041180121. Prevention for Recurrence Of The preEClampsia with TAdalafil [A study on Prevention of Hypertensive Disorders of Pregnancy who have a history of severe hypertensive disorders of pregnancy -Pre-test for multicenter trial- - PROTECTA]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-jRCTs041180121 (first received 2019). [CENTRAL: CN-01972419]
Khachaturyan 2011 {published data only}
-
- Khachaturyan L, Groten T, Kahler C, Bulagay-Morschel M, Schneider U, Schleussner E. Reduction of intrauterine growth retardation (IUGR) in a high-risk group with the NO-donor penterythriltetranitrat (PETN) - A prospective randomized double-blind placebo trial. Geburtshilfe und Frauenheilkunde 2011;71(10):895. [CENTRAL: CN-01034461] [EMBASE: 70703843]
Lees 1998 {published data only}
-
- Lees C, Valensise H, Black R, Harrington K, Byiers S, Romanini C, et al. The efficacy and fetal-maternal cardiovascular effects of transdermal glyceryl trinitrate in the prophylaxis of pre-eclampsia and its complications: a randomized double-blind placebo-controlled trial. Ultrasound in Obstetrics & Gynecology 1998;12(5):334-8. [CENTRAL: CN-00157005] [PMID: ] - PubMed
Lopez‐Molina 2008 {published data only}
-
- Lopez-Molina K, Gonzalez-Altamirano JC, Valdivia-Silva JE. Early L-arginine therapy improves notably the fetal growth in preeclamptic women. A randomized controlled trial. In: 55th Annual Meeting of the Society of Gynecologic Investigation; 2008 March 26-29; San Diego, USA. 2008:Abstract no: 801. [CENTRAL: CN-00653551]
Madhubala 2006 {published data only}
-
- Madhubala M. Use of L. Arginine in oligohydramnios [abstract]. In: 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6-9; Cochin, Kerala State, India. 2006:42. [CENTRAL: CN-00582409]
Monari 2021 {published data only}
-
- Monari F, Menichini D, Pignatti L, Basile L, Facchinetti F, Neri I. Effect of L-Arginine supplementation in pregnant women with chronic hypertension and previous placenta vascular disorders receiving aspirin prophylaxis: a randomized control trial. Minerva Obstetrics and Gynecology 2021;73:782-9. [CENTRAL: CN-02279076] [PMID: ] - PubMed
NCT01355822 {published data only}
-
- NCT01355822. Impact of the NO-donor Pentaerythrithyltetrantrate on perinatal outcome in high-risk pregnancies [Impact of the NO-donor Pentaerythrithyltetrantrate on Perinatal Outcome in High-risk Pregnancies: a Prospective Randomized Pilot Study]. Https://clinicaltrials.gov/show/NCT01355822 (first received 2011 May 16). [CENTRAL: CN-01486553]
NCT02782559 {published data only}
-
- NCT02782559. Efficacy of sildenafil in preterm preeclampsia. Https://clinicaltrials.gov/show/NCT02782559 (first received 11 February 2016). [CENTRAL: CN-01558533]
NCT02801695 {published data only}
-
- NCT02801695. Evaluation of the efficacy of citrulline supplementation on the delay of delivery for women hospitalized for pre-eclampsia (CITRUPE). Https://clinicaltrials.gov/show/nct02801695 (first received 2016 Jun 3). [CENTRAL: CN-01578215]
NCT03262961 {published data only}
-
- NCT03262961. Use of sildenafil citrate in management of mild pre-eclampsia [Use of Sildenafil Citrate in Management of Mild Pre-eclampsia: A Randomized Controlled Trial]. Https://clinicaltrials.gov/show/nct03262961 (first received 19 August 2017). [CENTRAL: CN-01586413]
NCT03669185 {published data only}
-
- NCT03669185. Pentaerithrityl Tetranitrate (PETN) for secondary prevention of intrauterine growth restriction [Pentaerithrityltetranitrat (PETN) Zur Sekundarprophylaxe Der Intrauterinen Wachstumsretardierung]. Https://clinicaltrials.gov/show/nct03669185 (first received 2018 Sep 13). [CENTRAL: CN-01663222]
Neri 2010 {published data only}
-
- Neri I, Monari F, Sgarbi L, Berardi A, Masellis G, Facchinetti F. L-arginine supplementation in women with chronic hypertension: impact on blood pressure and maternal and neonatal complications. Journal of Maternal-fetal & Neonatal Medicine 2010;23(12):1456-60. [CENTRAL: CN-00772723] [PMID: ] - PubMed
Picciolo 2000 {published data only}
-
- Picciolo C, Roncaglia N, Neri I, Pasta F, Arreghini A, Facchinetti F. Nitric oxide in the prevention of pre-eclampsia. Prenatal and Neonatal Medicine 2000;5(4):212-5. [CENTRAL: CN-00399568] [EMBASE: 30809202]
Razik 2016 {published data only}
-
- Razik MA, El-Berry S, Abosereah M, Edris Y, Sharafeldeen A. Prophylactic treatment for preeclampsia in high-risk teenage primigravidae with nitric oxide donors: a pilot study. Journal of Maternal-fetal & Neonatal Medicine 2016;29(16):2617-20. [CENTRAL: CN-01264685] [PMID: ] - PubMed
Reyna‐Villasmil 2001 {published data only}
-
- Reyna-Villasmil E, Prieto-Franchi M, Guerra-Velazquez M, Torres-Montilla M. Effect of transdermal nitroglycerin on umbilical artery blood flow in preeclampsia [abstract]. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 2):486. [CENTRAL: CN-00363873]
Rytewski 2005 {published data only}
-
- Rytlewski K, Olszanecki R, Korbut R, Zdebski Z. Effects of prolonged oral supplementation with L-arginine on blood pressure and nitric oxide synthesis in preeclampsia. European Journal of Clinical Investigation 2005;35(1):32-7. [CENTRAL: CN-00575546] [EMBASE: 40187952] [PMID: ] - PubMed
Samangaya 2009a {published data only}
-
- Samangaya RA, Wareing M, Skillern L, Baker PN. Phosphodiesterase inhibitor effect on small artery function in preeclampsia. Hypertension in Pregnancy 2011;30(2):144-52. [CENTRAL: CN-00783854] - PubMed
Samangaya 2009b {published data only}
-
- Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, et al. A randomised, double-blinded, placebo-controlled trial of the phosphodiesterase type 5 inhibitor sildenafil in the treatment of preeclampsia. Hypertension in Pregnancy 2009;28:369-82. [CENTRAL: CN-00740358] - PubMed
Schlembach 2013 {published data only}
-
- Schlembach D, Groten T, Lehmann T, Schneider U, Schleussner E. Impact of the NO-donor pentaerythrithyltetranitrate on perinatal outcome in high-risk pregnancies: a prospective randomized double blinded pilot study. Pregnancy Hypertension 2013;3(2):62. [CENTRAL: CN-02093670] - PubMed
Schleussner 2014 {published data only}
-
- Schleussner E, Kaehler C, Bulgay-Moerschel M, Schneider U, Hoyer H, Groten T. Reduction of intrauterine growth retardation (IUGR) in a high-risk group with the no-donor penterythriltetranitrat (PETN) - a prospective randomized double-blind placebo trial. Placenta 2010;31(9):A.82. [CENTRAL: CN-00795536]
-
- Schleussner E, Lehmann T, Kahler C, Schneider U, Schlembach D, Groten T. Erratum: Impact of the nitric oxide-donor pentaerythrityl-tetranitrate on perinatal outcome in risk pregnancies: A prospective, randomized, double-blinded trial (Journal of Perinatal Medicine 42:4 (507-514) DOI: 10.1515/jpm-2013-0212). Journal of Perinatal Medicine 2015;43(5):641. [CENTRAL: CN-01129520] [EMBASE: 605946537] [PMID: ] - PubMed
-
- Schleussner E, Lehmann T, Kahler C, Schneider U, Schlembach D, Groten T. Impact of the nitric oxide-donor pentaerythrityl-tetranitrate on perinatal outcome in risk pregnancies: a prospective, randomized, double-blinded trial. Journal of Perinatal Medicine 2014;42(4):507-14. [CENTRAL: CN-00999631] [EMBASE: 2014468781] [PMID: ] - PubMed
Staff 2004 {published data only}
-
- Staff AC, Berge L, Haugen G, Lorentzen B, Mikkelsen B, Henriksen T. Dietary supplementation with L-arginine or placebo in women with pre-eclampsia. Acta Obstetricia et Gynecologica Scandinavica 2004;83(83):103-7. [CENTRAL: CN-00459625] [PMID: ] - PubMed
Tan 2000 {published data only}
-
- Tan Y, Zhang W, Lu B. Treatment of intrauterine growth retardation with magnesium sulfate. Zhonghua Fu Chan Ke za Zhi 2000 Nov;35(11):664-6. [CENTRAL: CN-00408397] [PMID: ] - PubMed
Teichert 2019 {published data only}
-
- Teichert V, Gutierrez-Samudio R, Pastuschek J, Markert U, Groten T. PETN induced HO-1 expression in endothelial cells as a target for secondary prevention of endothelial dysfunction. Placenta 2019;83:e80. [CENTRAL: CN-01999164] [EMBASE: 2002510299]
Trapani 2016b {published data only}
-
- RBR-8qj4p5. Evaluation of blood pressure, blood flow fetal and neonatal outcome with the use of a phosphodiesterase type 5 inhibitor in the treatment of women with preeclampsia [Hemodynamics and perinatal assessment of phosphodiesterase type 5 inhibitors in pregnant women with preeclampsia]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=RBR-8qj4p5 (first received 2016). [CENTRAL: CN-01841864]
-
- Trapani A, Goncalves LF, Trapani TF, Vieira S, Pires M, Pires MM. Perinatal and hemodynamic evaluation of sildenafil citrate for preeclampsia treatment: a randomized controlled trial. Obstetrics and Gynecology 2016;128(2):253-9. [CENTRAL: CN-01379691] [PMID: ] - PubMed
-
- Vigil-De Gracia P, Ludmir J. Perinatal and hemodynamic evaluation of sildenafil citrate for preeclampsia treatment: a randomized controlled trial. Obstetrics and Gynecology 2016;128(5):1181-2. [PMID: ] - PubMed
Valdivia‐Silva 2009 {published data only}
-
- Valdivia-Silva JE, Lopez-Molina K, Macedo R. Effect of early L-arginine therapy on intrauterine growth restriction in preeclampsia. A randomized controlled trial in Latin-American women. [Spanish] [Efecto de la terapia temprana con L-arginina en el crecimiento intrauterino restringido en la preclampsia. Estudio aleatorizado en mujeres latinoamericanas]. Progresos en Obstetricia y Ginecologia 2009;52(2):89-98. [CENTRAL: CN-00754747] [EMBASE: 354306780]
Valensise 2005 {published data only}
-
- Valensise H, Vasapollo B, Novelli GP, Altomare F, Arduini D. Nitric oxide donors and fluid therapy increase fetal growth in gestational hypertension [abstract]. Ultrasound in Obstetrics & Gynecology 2005;26(4):440. [CENTRAL: CN-00550253]
Xiao 2005 {published data only}
-
- Xiao XM, Li LP. L-arginine treatment for asymmetric fetal growth restriction. International Journal of Gynaecology and Obstetrics 2005;88(1):15-8. [CENTRAL: CN-00550215] - PubMed
References to studies awaiting assessment
CTRI/2019/09/021382 {published data only}
-
- CTRI/2019/09/021382. Treatment of intra uterine growth retardation using vidaryadi ghrita and L-Arginne. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2019/09/021382 (first received 2019). [CENTRAL: CN-02349424]
CTRI/2022/03/041053 {published data only}
-
- CTRI/2022/03/041053. Evaluate the effect of Madhumalinivasanta rasa along with Yastimadhuadi siddha ghrita matra basti in Upavistaka(intra-uterine growth restriction) compaire with L-arginine sachet. https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2022/03/041053 (first received 2022). [CENTRAL: CN-02409098]
Dastjerdi 2012a {published data only}
El‐Sayed 2018b {published data only}
-
- El-Sayed MA, Saleh SA, Maher MA, Khidre AM. Utero-placental perfusion Doppler indices in growth restricted fetuses: effect of sildenafil citrate. Journal of Maternal-fetal & Neonatal Medicine 2018;31(8):1045-50. [CENTRAL: CN-01628070] [PMID: ] - PubMed
-
- NCT02362399. Effect of Sildenafil citrate on uteri- placental perfusion, Doppler indices in growth restricted fetuses [Effect of Sildenafil Citrate on Uteri- Placental perfusion, Doppler indices in growth restricted fetuses]. Https://clinicaltrials.gov/show/NCT02362399 (first received 2015 Feb 5). [CENTRAL: CN-01552114]
El‐Shalakany 2018 {published data only}
-
- El-Shalakany A, Abd El Aleem MM, Zaifer M, Bawady A. Sildenafil citrate and uteroplacental perfusion in fetal growth restriction. Egyptian Journal of Hospital Medicine 2018;71(4):2989-95.
Eshraghi 2021 {published data only}
-
- Eshraghi N, Mohamadianamiri M, Ebrahimi M, Karimi F. The effect of sildenafil on intrauterine growth restriction (IUGR) of fetus with gestational age above 28 weeks and neonatal outcomes. International Journal of Pediatrics 2021;9(6):13643-51.
-
- TCTR20200526006. The effect of sildenafil on intrauterine growth restriction (IUGR) of fetus with gestational age above 28 weeks and neonatal outcomes [Effect of Sildenafil on Intrauterine Growth Restriction (IUGR) of Fetus]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20200526006 (first received 2020). [CENTRAL: CN-02189826]
Gupta 2017 {published data only}
-
- Gupta S, Chauhan M, Sen J, Nanda S. Effect of transdermal nitroglycerine on doppler velocity waveforms of the uterine, umbilical and fetal middle cerebral arteries in patients with chronic placental insufficiency: a prospective RCT. Journal of Clinical and Diagnostic Research : JCDR 2017;11(7):QC13-7. [CENTRAL: CN-01403941] [EMBASE: 617499160] - PMC - PubMed
Huras 2014 {published data only}
-
- Huras H, Kalinka J, Radon-Pokracka M, Kusmierska-Urban K, Kufelnicka-Babout M, Nowak M, et al. Effects of pentoxifylline and docosahexaenoic acid supplemental treatment in intrauterine growth restriction. Journal of Maternal-fetal & Neonatal Medicine 2014;27(Suppl 1):131. [CENTRAL: CN-01064457] [EMBASE: 71504804]
Naseef 2022 {published data only}
-
- Naseef P, El Fatah IA, Tharwat A, El Sayed M. Difference between oral isosorbide mononitrate & sildenafil citrate therapy in reducing umbilical artery doppler indices in pregnancies with fetal growth restriction; a prospective randomized control trial. Egyptian Journal of Hospital Medicine 2022;88(1):2958-63.
NCT01107782 {published data only}
-
- NCT01107782. Sildenafil and uteroplacental perfusion [Phase 2 Study of Fetal Growth Retardation Treatment by Sildenafil]. Https://clinicaltrials.gov/show/NCT01107782 (first received 2010 Jan 13). [CENTRAL: CN-01529139]
NCT02590536 {published data only}
-
- NCT02590536. A trial evaluating the role of sildenafil in the treatment of fetal growth restriction [A randomized controlled trial evaluating the role of sildenafil in the treatment of fetal growth restriction]. Https://clinicaltrials.gov/show/NCT02590536 (first received 2015 Oct 21). [CENTRAL: CN-01493354]
PACTR201705002278236 {published data only}
-
- PACTR201705002278236. Sildenafil citrate for the treatment of asymmetrical intrauterine growth restriction: a randomized controlled trial. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201705002278236 (first received 2017). [CENTRAL: CN-01895111]
Rasheedy 2019 {published data only}
-
- NCT03230162. Sildenafil versus low molecular weight heparin in fetal growth restriction treatment. https://clinicaltrials.gov/show/NCT03230162 (first received 2017 July 26). [CENTRAL: CN-02420461]
-
- Rasheedy R, El Bishry G, Tarek R. Maternal low molecular weight heparin versus sildenafil citrate for fetal growth restriction: a randomized, parallel groups, open-label clinical trial. Journal of Perinatology 2019;40(5):715-23. [CENTRAL: CN-02008477] [EMBASE: 2003558866] [PMID: ] - PubMed
Serey 1980 {published data only}
-
- Serey C, Saling E. Human placental lactogen (HPL) levels in the maternal serum following long-term intake of xantinol nicotinate (Complamin Retard) [Hpl-verhalten im mutterlichen serum nach langzeitiger gabe von xantinol-nicotinat (complamin retard)]. Zeitschrift fur Geburtshilfe und Perinatologie 1980;184:283-9. [CENTRAL: CN-00465639] - PubMed
Shehata 2018 {published data only}
-
- NCT03153215. Sildenafil in severe intrauterine growth retardation [Evaluation of addition of Sildenafil Citrate for treatment of severe intrauterine growth restriction]. Https://clinicaltrials.gov/show/nct03153215 (first received 2017 May 5). [CENTRAL: CN-01575093]
-
- Shehata NA, Ali HA, Fahim AS, Katta MA, Hussein GK. Addition of sildenafil citrate for treatment of severe intrauterine growth restriction: a double blind randomized placebo controlled trial. Journal of Maternal-fetal & Neonatal Medicine 2018;33(10):1631-7. [CENTRAL: CN-01650710] [EMBASE: 624557684] - PubMed
Shen 2011 {published data only}
-
- Shen SF, Hua CH. Effect of L-arginine on the expression of Bcl-2 and Bax in the placenta of fetal growth restriction. Journal of Maternal-fetal & Neonatal Medicine 2011;24(6):822-6. [CENTRAL: CN-00793049] [PMID: ] - PubMed
Singh 2015 {published data only}
Singh 2018 {published data only}
-
- Singh R, Singh S, Yadav P, Singh H, Gupta S, Vardhan S. A comparative study to assess the efficacy of sildenafil citrate and l-arginine in the management of fetal growth restriction. Journal of SAFOG 2018;10(3):170-4. [CENTRAL: CN-01976232] [EMBASE: 2002347840]
Yadav 2021 {published data only}
-
- Yadav R, Yadav A, Kumari A. Role of sildenafil citrate therapy in early-onset fetal growth restriction. Journal of SAFOG 2021;13(6):392-5. [CENTRAL: CN-02387603] [EMBASE: 2015926791]
Zhang 2007 {published data only}
-
- Zhang N, Xiong AH, Xiao X, Li LP. Effect and mechanism of L-arginine therapy for fetal growth retardation due to pregnancy-induced hypertension. Nan Fang Yi Ke da Xue Xue Bao [Journal of Southern Medical University] 2007 Feb;27(2):198-200. [CENTRAL: CN-00627113] [PMID: ] - PubMed
References to ongoing studies
IRCT20160524028038N6 {published data only}
-
- IRCT20160524028038N6. Sildenafil citrate with melatonin and intrauterine growth restriction [Evaluation of the effect of Sildenafil Citrate with melatonin on intrauterine growth restriction]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20160524028038N6 (first received 2020). [CENTRAL: CN-02170379]
IRCT20210730052014N1 {published data only}
-
- IRCT20210730052014N1. Comparison of the effect of pomegranate juice with sildenafil in the treatment of intrauterine growth restriction. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20210730052014N1 (first received 2021). [CENTRAL: CN-02351408]
ISRCTN32082979 {published data only}
-
- ISRCTN32082979. Treatment of restriction in fetal growth with L-arginine [Treatment of restriction in fetal growth with L-arginine: randomized clinical trial, double-blind, controlled with placebo]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN32082979 (first received 2018). [CENTRAL: CN-01951140]
Maki 2022 {published data only}
-
- Maki S, Tanaka H, Takakura S, Nii M, Tanaka K, Ogura T, et al. Tadalafil treatment for fetuses with early-onset growth restriction: a protocol for a multicentre, randomised, placebo-controlled, double-blind phase II trial (TADAFER IIb). BMJ Open 2022;12(6):e054925. [CENTRAL: CN-02413174] [PMID: ] - PMC - PubMed
-
- jRCTs041190065. Tadalafil for fetus with early-onset growth restriction (TADAFER IIb) [The clinical trial of tadalafil treatment for fetus with early-onset growth restriction placebo-controlled ramdomized multicenter phase II trial- - TADAFER IIb]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-jRCTs041190065 (first received 2019). [CENTRAL: CN-01970876]
NCT0157521 {published data only}
-
- NCT00157521. L-Arginine in Pre-eclampsia [A Double-Blind, Randomized, Pilot Study to Explore Whether Enhancing L-Arginine Bioavailability by Oral Supplementation Increases NO Production and Prevents Peroxynitrite Generation in the Pre-Eclamptic Placenta]. Https://clinicaltrials.gov/show/nct00157521 (first received 2005 Sep 8). [CENTRAL: CN-01585481]
NCT02678221 {published data only}
-
- NCT02678221. Sildenafil citrate for the management of asymmetrical intrauterine growth restriction. Https://clinicaltrials.gov/show/NCT02678221 (first received 2016 February 09). [CENTRAL: CN-01555684]
NCT03177824 {published data only}
-
- NCT03177824. Sildenafil citrate for treatment of growth-restricted fetuses [Sildenafil Citrate for Treatment of Growth-restricted Fetuses]. Https://clinicaltrials.gov/show/nct03177824 (first received 2017 Apr 26). [CENTRAL: CN-01594727]
NCT03321292 {published data only}
-
- NCT03321292. L-arginine in treatment of intrauterine growth restriction [Effect of L-Arginine on Intrauterine Growth Restriction Fetuses Measured by Birth Weight: Randomized Controlled Trial]. Https://clinicaltrials.gov/show/NCT03321292 (first received 2017 Sep 28). [CENTRAL: CN-01565163]
NCT05029778 {published data only}
-
- NCT05029778. Arginine + citrulline as a supplement for weight gain in fetus with a decrease in their growth curve [Efficacy L-arginine + L-citrulline as a Dietary Supplement vs Placebo for Weight Gain in Fetus With a Decrease in Their Growth Curve in the Third Trimester of Pregnancy]. Https://clinicaltrials.gov/show/NCT05029778 (first received 2021 September 01). [CENTRAL: CN-02307577]
Umekawa 2018 {published data only}
UMIN000023778 {published data only}
-
- UMIN000023778. A multicenter phase II trial of the efficacy and safety of tadalafil in fetus with early-onset growth restriction. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000023778 (first received 2016). [CENTRAL: CN-01832739]
Additional references
Barnes 2019
Bourdon 2016
-
- Bourdon A, Parnet P, Nowak C, Tran NT, Winer N, Darmaun D. L-Citrulline supplementation enhances fetal growth and protein synthesis in rats with intrauterine growth restriction. Journal of Nutrition 2016;146(3):532-41. - PubMed
Buhimschi 1998
-
- Buhimschi IA, Saade GR, Chwalisz K, Garfield RE. The nitric oxide pathway in pre-eclampsia: pathophysiological implications. Human Reproduction Update 1998;4(1):25-42. - PubMed
Carlisle 2017
-
- Carlisle JB. Data fabrication and other reasons for non-random sampling in 5087 randomised, controlled trial in anaesthetic and general medical journals. Anaesthesia 2017;72:944-52. - PubMed
Casanello 2002
-
- Casanello P, Sobrevia L. Intrauterine growth retardation is associated with reduced activity and expression of the cationic amino acid transport systems y+/hCAT-1 and y+/hCAT-2B and lower activity of nitric oxide synthase in human umbilical vein endothelial cells. Circ ulation Research 2002 Jul 26;91(2):127-34. - PubMed
Chen 2016
Choudhary 2016
Conde‐Agudelo 2013
-
- Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for predicting intrauterine growth restriction: a systematic review and meta-analysis. British Journal of Obstetrics and Gynaecology 2013;120(6):681-94. - PubMed
Dastjerdi 2012
Dunn 2017
-
- Dunn L, Greer R, Flenady V, Kumar S. Sildenafil in pregnancy: a systematic review of maternal tolerance and obstetric and perinatal outcomes. Fetal Diagnosis and Therapy 2017;41(2):81-8. - PubMed
El‐Sayed 2018
-
- El-Sayed MA, Saleh SA, Maher MA, Khidre AM. Utero-placental perfusion Doppler indices in growth restricted fetuses: effect of sildenafil citrate. Journal of Maternal-fetal & Neonatal Medicine 2018;31(8):1045-50. - PubMed
Everett 2014
Gordijn 2016
-
- Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound in Obstetrics & Gynecology 2016;48(3):333-9. - PubMed
Grimble 2007
-
- Grimble GK. Adverse gastrointestinal effects of arginine and related amino acids. Journal of Nutrition 2007;137(6 (Suppl 2)):1693S-1701S. - PubMed
GRIT study group 2003
-
- GRIT study group. A randomised trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG: an international journal of obstetrics and gynaecology 2003;110(1):27-32. - PubMed
Groom 2018
-
- Groom KM, David AL. The role of aspirin, heparin, and other interventions in the prevention and treatment of fetal growth restriction. American Journal of Obstetrics and Gynecology 2018;218(2S):S829-S840. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Johal 2014
Katarzyna 2013
Krause 2011
Latini 2008
-
- Latini G, Rosati E, De Felice C, Del Vecchio A. Sildenafil administration to a patient with refractory persistent pulmonary hypertension of the newborn. Journal of Maternal-fetal & Neonatal Medicine 2008;21(9):671-3. - PubMed
Lees 2013
-
- Lees C, Marlow N, Arabin B, Bilardo CM, Brezinka C, Derks JB, et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound in Obstetrics & Gynecology 2013;42(4):400-8. - PubMed
Lin 2012
-
- Lin TH, Su YN, Shih JC, Hsu HC, Lee CN. Resolution of high uterine artery pulsatility index and notching following sildenafil citrate treatment in a growth-restricted pregnancy. Ultrasound in Obstetrics & Gynecology 2012;40(5):609-10. - PubMed
Nardozza 2017
-
- Nardozza LM, Caetano AC, Zamarian AC, Mazzola JB, Silva CP, Marcal VM, et al. Fetal growth restriction: current knowledge. Archives of Gynecology and Obstetrics 2017;295(5):1061-77. - PubMed
Oyston 2015
-
- Oyston CJ, Stanley JL, Baker PN. Potential targets for the treatment of preeclampsia. Expert Opinion on Therapeutic Targets 2015;19(11):1517-30. - PubMed
Paauw 2017
-
- Paauw ND, Terstappen F, Ganzevoort W, Joles JA, Gremmels H, Lely AT. Sildenafil during pregnancy: a preclinical meta-analysis on fetal growth and maternal blood pressure. Hypertension 2017;70(5):998-1006. - PubMed
Panda 2014
Papageorghiou 2004
-
- Papageorghiou AT, Yu CK, Nicolaides KH. The role of uterine artery Doppler in predicting adverse pregnancy outcome. Best Practice & Research. Clinical Obstetrics & Gynaecology 2004;18(3):383-96. - PubMed
Papageorghiou 2007
-
- Papageorghiou AT, Leslie K. Uterine artery Doppler in the prediction of adverse pregnancy outcome. Current Opinion in Obstetrics and Gynecology 2007;19(2):103-9. - PubMed
Refuerzo 2006
-
- Refuerzo JS, Sokol RJ, Aranda JV, Hallak M, Hotra JW, Kruger M, et al. Sildenafil citrate and fetal outcome in pregnant rats. Fetal Diagnosis and Therapy 2006;21(3):259-63. - PubMed
RevMan 2014 [Computer program]
-
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Samangaya 2009
-
- Samangaya RA, Mires G, Shennan A, Skillern L, Howe D, McLeod A, et al. A randomised, double-blinded, placebo-controlled study of the phosphodiesterase type 5 inhibitor sildenafil for the treatment of preeclampsia. Hypertension in Pregnancy 2009;28(4):369-82. - PubMed
Sanchez‐Aparicio 2008
-
- Sanchez-Aparicio P, Mota-Rojas D, Nava-Ocampo AA, Trujillo-Ortega ME, Alfaro-Rodriguez A, Arch E, et al. Effects of sildenafil on the fetal growth of guinea pigs and their ability to survive induced intrapartum asphyxia. American Journal of Obstetrics and Gynecology 2008;198(1):127 e1-6. - PubMed
Satterfield 2010
-
- Satterfield MC, Bazer FW, Spencer TE, Wu G. Sildenafil citrate treatment enhances amino acid availability in the conceptus and fetal growth in an ovine model of intrauterine growth restriction. Journal of Nutrition 2010;140(2):251-8. - PubMed
Severi 2000
-
- Severi FM, Rizzo G, Bocchi C, D'Antona D, Verzuri MS, Arduini D. Intrauterine growth retardation and fetal cardiac function. Fetal Diagnosis and Therapy 2000;15(1):8-19. - PubMed
Smith 2006
-
- Smith HA, Canter JA, Christian KG, Drinkwater DC, Scholl FG, Christman BW, et al. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. Journal of Thoracic and Cardiovascular Surgery 2006;132(1):58-65. - PubMed
Stanley 2012
-
- Stanley JL, Andersson IJ, Poudel R, Rueda-Clausen CF, Sibley CP, Davidge ST, et al. Sildenafil citrate rescues fetal growth in the catechol-O-methyl transferase knockout mouse model. Hypertension 2012;59(5):1021-8. - PubMed
Sun 2014
-
- Sun X, Wang K, Wang W, Li B. [Clinical study on sildenafil in treatment of pregnant women with pulmonary arterial hypertension]. Zhonghua Fu Chan Ke Za Zhi 2014;49(6):414-8. - PubMed
Tran 2017
-
- Tran NT, Amarger V, Bourdon A, Misbert E, Grit I, Winer N, et al. Maternal citrulline supplementation enhances placental function and fetal growth in a rat model of IUGR: involvement of insulin-like growth factor 2 and angiogenic factors. Journal of Maternal-fetal & Neonatal Medicine 2017;30(16):1906-11. - PubMed
Trapani 2016c
-
- Trapani A Jr, Goncalves LF, Trapani TF, Vieira S, Pires M, Pires MM. Perinatal and hemodynamic evaluation of sildenafil citrate for preeclampsia treatment: a randomized controlled trial. Obstetrics and Gynecology 2016;128(2):253-9. - PubMed
Trapani 2016d
-
- Trapani A Jr, Goncalves LF, Trapani TF, Franco MJ, Galluzzo RN, Pires MM. Comparison between transdermal nitroglycerin and sildenafil citrate in intrauterine growth restriction: effects on uterine, umbilical and fetal middle cerebral artery pulsatility indices. Ultrasound in Obstetrics & Gynecology 2016;48(1):61-5. - PubMed
Turner 2022
-
- Turner JM, Russo F, Deprest J, Mol BW, Kumar S. Phosphodiesterase-5 inhibitors in pregnancy: Systematic review and meta-analysis of maternal and perinatal safety and clinical outcomes. British Journal of Obstetrics & Gynaecology 2022;Online ahead of print:Online ahead of print. - PubMed
von Dadelszen 2011
-
- Dadelszen P, Dwinnell S, Magee LA, Carleton BC, Gruslin A, Lee B, et al. Sildenafil citrate therapy for severe early-onset intrauterine growth restriction. BJOG: an international journal of obstetrics and gynaecology 2011;118(5):624-8. - PubMed
Wareing 2005
-
- Wareing M, Myers JE, O'Hara M, Baker PN. Sildenafil citrate (Viagra) enhances vasodilatation in fetal growth restriction. Journal of Clinical Endocrinology and Metabolism 2005;90(5):2550-5. - PubMed
Wu 2009
-
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids 2009;37(1):1-17. - PubMed
References to other published versions of this review
Pels 2020
-
- Pels A, Kenny L, Baker P, Dadelszen P, Gludd C, Kariya C, et al. Interventions affecting the nitric oxide pathway versus placebo or no therapy for fetal growth restriction in pregnancy. PROSPERO 2020 CRD42020158093 Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020158093. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

