Fucoidan and microtopography on polyvinyl alcohol hydrogels guided axons and enhanced neuritogenesis of pheochromocytoma 12 (PC12) cells
- PMID: 37429292
- PMCID: PMC10364238
- DOI: 10.1088/1748-605X/ace5fe
Fucoidan and microtopography on polyvinyl alcohol hydrogels guided axons and enhanced neuritogenesis of pheochromocytoma 12 (PC12) cells
Abstract
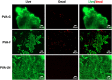
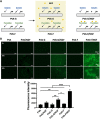
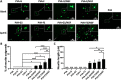
Artificial nerve grafts that support axon growth hold promises in promoting nerve regeneration and function recovery. However, current artificial nerve grafts are insufficient to regenerate axons across long nerve gaps. Specific biochemical and biophysical cues are required to be incorporated to artificial nerve grafts to promote neural cell adhesion and guide neurite outgrowth. Polyvinyl alcohol (PVA) nerve conduits have been clinically approved, but the applicability of PVA nerve conduits is limited to short injuries due to low cell binding. In this study, we explored the incorporation of biochemical cues and topographical cues for promoting neuritogenesis and axon guidance. PVA was conjugated with extracellular matrix proteins and fucoidan, a bioactive sulfated polysaccharide, to improve cell adhesion. Micro-sized topographies, including 1.8 μm convex lenses, 2 μm gratings, and 10 μm gratings were successfully fabricated on PVA by nanofabrication, and the synergistic effects of topography and biochemical molecules on pheochromocytoma 12 (PC12) neuritogenesis and neurite alignment were studied. Conjugated fucoidan promoted the percentage of PC12 with neurite outgrowth from 0% to 2.8% and further increased to 5% by presenting laminin on the surface. Additionally, fucoidan was able to bind nerve growth factor (NGF) on the surface and allow for PC12 to extend neurites in NGF-free media. The incorporation of 2 μm gratings could double the percentage of PC12 with neurite outgrowth and neurite length, and guided the neurites to extend along the grating axis. The work presents a promising strategy to enhance neurite formation and axon guidance, presenting significant value in promoting nerve regeneration.
Keywords: artificial nerve graft; axon guidance; fucoidan; neuritogenesis; polyvinyl alcohol; topography.
Creative Commons Attribution license.
Figures





Similar articles
-
Extracellular matrix allows PC12 neurite elongation in the absence of microtubules.J Cell Biol. 1990 Jan;110(1):71-9. doi: 10.1083/jcb.110.1.71. J Cell Biol. 1990. PMID: 2153148 Free PMC article.
-
Neurite outgrowth and protein synthesis by PC12 cells as a function of substratum and nerve growth factor.J Neurosci. 1982 Aug;2(8):1157-75. doi: 10.1523/JNEUROSCI.02-08-01157.1982. J Neurosci. 1982. PMID: 7108587 Free PMC article.
-
Micropatterned biodegradable polyesters clicked with CQAASIKVAV promote cell alignment, directional migration, and neurite outgrowth.Acta Biomater. 2018 Jul 1;74:143-155. doi: 10.1016/j.actbio.2018.05.018. Epub 2018 May 13. Acta Biomater. 2018. PMID: 29768188
-
Topography, cell response, and nerve regeneration.Annu Rev Biomed Eng. 2010 Aug 15;12:203-31. doi: 10.1146/annurev-bioeng-070909-105351. Annu Rev Biomed Eng. 2010. PMID: 20438370 Free PMC article. Review.
-
Microfluidic Systems for Neural Cell Studies.Bioengineering (Basel). 2023 Jul 30;10(8):902. doi: 10.3390/bioengineering10080902. Bioengineering (Basel). 2023. PMID: 37627787 Free PMC article. Review.
Cited by
-
Release of delta-9-tetrahydrocannabinol from polyvinyl alcohol hydrogels and its safe interaction with human skin fibroblasts.Front Drug Deliv. 2024 Jan 31;4:1303812. doi: 10.3389/fddev.2024.1303812. eCollection 2024. Front Drug Deliv. 2024. PMID: 40836986 Free PMC article.
-
Research progress on composite nerve guidance conduits with immune-regulatory functions.Front Immunol. 2025 Jun 10;16:1622508. doi: 10.3389/fimmu.2025.1622508. eCollection 2025. Front Immunol. 2025. PMID: 40557153 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
